Industries > Pharma > Global Vaccine Contract Manufacturing Market Report 2018-2028
Global Vaccine Contract Manufacturing Market Report 2018-2028
Leading Countries, Technologies and Companies
The global vaccine contract manufacturing market was worth $883.0m in 2017 and is expected to grow at a CAGR of 9.1% from 2017-2022. Visiongain predicts a CAGR of 9.2% for the global vaccine contract manufacturing market over the whole forecast period (2017-2028).
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in this sector.
In this brand new 150-page report you will receive 69 charts– all unavailable elsewhere.
The 150-page report provides clear detailed insight into the global vaccine contract manufacturing market. Discover the key drivers and challenges affecting the market.
By ordering and reading our brand new report today you stay better informed and ready to act.
Report Scope
• Revenue and growth forecasts to 2028 for the global vaccine market and market shares in 2017 and 2018 of the submarkets: paediatric vaccines, adult vaccines, influenza vaccines and therapeutic vaccines
• Revenue and growth forecasts to 2028 for the global vaccine contract manufacturing market
• Revenue and growth forecasts to 2028 for the leading national markets:
• United States
• Germany
• France
• United Kingdom
• Italy
• Spain
• Japan
• Brazil
• Russia
• China
• India
• Rest of the World
• Discussion and profiles of the leading players in the vaccine contract manufacturing market:
• Baxter BioPharma Solutions
• Boehringer Ingelheim
• Catalent
• Charles River Laboratories
• IDT Biologika
• Lonza
• Meridian Life Science
• Sigma Aldrich Fine Chemicals
• Merck
• Synco Bio Partners
• Analysis of what stimulates and restrains the global vaccine contract manufacturing market: Trends and SWOT Analysis
Visiongain’s study is intended for anyone requiring commercial analyses for the global vaccine contract manufacturing market. You find data, trends and predictions.
Buy our report today Global Vaccine Contract Manufacturing Market Report 2018-2028: Leading Countries, Technologies and Companies.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
1. Report Overview
1.1 World Vaccine Contract Manufacturing Market Highlights
1.2 Why You Should Read This Report?
1.3 How This Report Delivers
1.4 Key Questions Answered by This Analytical Report
1.5 Who is This Report For?
1.6 Methodology
1.7 Associated Visiongain Reports
1.8 Frequently Asked Questions (FAQ)
2. Introduction to Vaccines
2.1 The History of Vaccines
2.2 How Do Vaccines Work?
2.2.1 Artificial Immunity
2.3 Types of Vaccine
2.3.1 Live, Attenuated Vaccines (LAVs)
2.3.2 Recombinant Live Vaccines
2.3.3 Inactivated Vaccines
2.3.4 Toxoid Vaccines
2.3.5 Subunit Vaccines
2.3.6 Conjugate Vaccines
2.3.7 Recombinant Subunit Vaccines
2.3.8 DNA Vaccines
2.3.9 Recombinant Vector Vaccines
2.3.10 Therapeutic Vaccines
2.4 Diseases Preventable with Vaccines
2.5 The Rise of the Vaccines Market
2.6 Technological and Regulatory Improvements
3. Vaccine Manufacturing Technologies, 2017
3.1 Summary of Vaccine Technology Trends
3.2 New Substrates for Vaccine Production
3.2.1 Shift towards Cell-Based Manufacturing Technology
3.2.2 Benefits of Cell-Based Techniques
3.2.3 Mammalian Cell Lines
3.2.3.1 MCDK (Madin Darby Canine Kidney Cells)
3.2.3.2 Vero Cells
3.2.3.3 PerC6 Cells
3.2.4 Avian-Derived Cell Lines
3.2.4.1 EB66 Stem Cell Technology: Vivalis
3.2.5 Plant-Based Vaccines
3.2.5.1 Medicago’s Proficia VLP Vaccine Technology
3.2.6 Insect Egg-Based Production Systems
3.2.6.1 Novavax
3.2.6.2 Protein Sciences Corporation (PSC)
3.3 Next-Generation Expression Systems and Vectors: Increasing Production Yield
3.3.1 AdVac Technology: Crucell
3.3.2 AdCEV Vectors: AfriVax
3.3.3 Pfenex Expression Technology: Pfenex
3.4 Equipment Trends
3.4.1 The Shift Towards Disposable Single-use Equipment
3.4.2 Bioreactors and Vaccine Production
3.4.2.1 Single-Use Bioreactors
3.4.2.2 Main Applications of Disposable Bioreactors
3.4.2.3 Current Single-Use Bioreactor Systems on the Market
3.5 Prefilled Syringes and Vaccines
3.5.1 Growing Market for Pre-Filled Syringes
3.5.2 Pre-Filled Vaccine Products
3.5.3 Drivers and Restraints for Pre-Filled Syringes
3.5.4 Product Stability and Quality Assurance Programme
3.5.4.1 Paediatric H1N1 Vaccine
3.5.4.2 Reported Challenges Flu Vaccines
3.5.4.3 Novartis' Agriflu and Fluad Ban is Lifted
3.5.4.4 Baxter Flu Vaccine and Side Effects
3.5.4.5 Reported Shelf Life with H1N1 Vaccine
3.6 Lyophilisation and Vaccine Manufacturing
3.6.1 Lyophilisation of Vaccines Will Increase
3.7 Cell Media Can Improve Virus Yield
4. The Global Vaccine Manufacturing Market 2018-2028
4.1 Overview of the Global Vaccine Manufacturing Market 2018-2028
4.2 World Vaccine Market Forecast 2018-2028
4.3 The Global Vaccine Market by Submarket, 2016 and 2017
4.4 Growth in Vaccine Market Will Drive Efficiency in Vaccine Manufacturing
4.5 Paediatric Vaccines Market Overview
4.6 Adult Prophylactic Vaccines Market Overview
4.7 Influenza Vaccines Market Overview
4.8 Therapeutic Vaccines Market Overview
5. The Global Vaccine Contract Manufacturing 2018-2028
5.1 Outsourcing in the Pharmaceutical and Biotechnology Industry
5.1.1 Reasons to Outsource
5.1.2 Benefits of Outsourcing
5.1.3 The Challenges in Outsourcing Vaccine Manufacturing
5.1.4 Why Should Companies Outsource Vaccine Manufacturing?
5.1.5 Strategic Outsourcing vs. Tactical Outsourcing
5.1.6 Virtual Companies
5.2 Contract Manufacturing Organisations (CMOs)
5.2.1 Manufacturing Services Offered by CMOs
5.2.2 Vaccine Manufacturing Activities Typically Outsourced
5.3 The Pharmaceutical Contract Manufacturing Market: Overall Revenue Forecast 2016-2028
5.4 Contract Manufacturing Market for Vaccines 2017-2028
5.4.1 Market Size for Contract Manufacturing of Vaccines 2017
5.4.2 Vaccine Contract Manufacturing Market Forecast 2017-2028
6. The Leading National Markets for Vaccine Contract Manufacturing, 2018-2028
6.1 The Leading National Markets for Vaccine Contract Manufacturing
6.2 The Leading National Markets for Vaccine Contract Manufacturing, 2016-2017
6.3 The Leading National Markets for Vaccine Contract Manufacturing, 2017-2028
6.3.1 The US Vaccine Contract Manufacturing Market Forecast, 2017-2028
6.3.2 The EU5 Vaccine Contract Manufacturing Market, 2016 and 2017
6.3.2.1 The EU5 Vaccine Contract Manufacturing Market, 2016-2028
6.3.2.2 The German Vaccine Contract Manufacturing Market, 2016-2028
6.3.2.3 The Italian Vaccine Contract Manufacturing Market, 2018-2028
6.3.2.4 The UK Vaccine Contract Manufacturing Market, 2018-2028
6.3.2.5 The French Vaccine Contract Manufacturing Market, 2018-2028
6.3.2.6 The Spanish Vaccine Contract Manufacturing Market, 2018-2028
6.3.3 The Japanese Vaccine Contract Manufacturing Market, 2018-2028
6.3.4 The Chinese Vaccine Contract Manufacturing Market, 2018-2028
6.3.5 The Indian Vaccine Contract Manufacturing Market, 2018-2028
6.3.6 The Russian Vaccine Contract Manufacturing Market, 2018-2028
6.3.7 The Brazilian Vaccine Contract Manufacturing Market, 2018-2028
7. Leading Vaccine Contract Manufacturing Companies, 2018-2028
7.1 Leading Vaccine Contract Manufacturing Organisations
7.2 Baxter BioPharma Solutions
7.3 Boehringer Ingelheim
7.3.1 Recent Financial Performance
7.3.2 Ben Venue Laboratories
7.3.3 Manufacturing Deals 2011-2017
7.4 Catalent
7.4.1 Recent Financial Performance
7.4.2 Catalent: Financial Performance by Segment, 2011-2016
7.4.3 Catalent Injectable Vaccines
7.4.4 Catalent: Zydis Technology
7.4.5 Regional Market Expansion Strategy
7.5 Charles River Laboratories
7.5.1 Recent Financial Performance, 2010-2016
7.5.2 Financial Performance by Segment, 2010-2016
7.5.3 Vaccine Manufacturing Services
7.5.4 Charles River Avian Vaccine Services
7.5.5 Vaccine Manufacturing Expansion
7.5.6 Outlook and Early Stage Restructuring
7.5.7 Partnerships
7.5.7.1 Partnership with AstraZeneca
7.5.7.2 Partnership with Batavia
7.6 IDT Biologika GmbH
7.7 Lonza
7.7.1 Recent Financial Performance
7.7.2 Manufacturing Division Restructuring
7.8 Meridian Life Science
7.8.1 Recent Financial Performance, 2010-2016
7.8.2 Recent Financial Performance by Segment, 2011-2016
7.9 Sigma-Aldrich Fine Chemicals (SAFC) (Merck KGaA)
7.10 SynCo Bio Partners
7.10.1 Recent Developments
7.10.1.1 Expansion of Facilities
7.10.1.2 FDA Approval of Partner Application
8. Vaccine Manufacturing Market: World Market Trends 2018-2028
8.1 Vaccine Contract Manufacturing Industry Trends
8.2 Strengths
8.2.1 Many Companies Cannot Afford In-house Capabilities
8.2.2 Outsourcing Improves Time to Market
8.2.3 Manufacturers Get Access to Specialised Technologies
8.2.4 Shift Towards Emerging Markets as Favourable Outsourcing Destinations
8.3 Weaknesses
8.3.1 Mass Vaccine Manufacturing Kept In-house
8.3.2 A Highly-Fragmented Vaccine Contract Manufacturing Market
8.3.3 An Unpredictable Supply and Demand Business Model
8.4 Opportunities
8.4.1 Many Vaccines in the Product Pipeline
8.4.2 Emerging Market’s Growing Demand for Vaccines
8.4.2.1 Dynamic Change: Emerging Markets are Major Vaccine Developers
8.4.3 Changing World Demographics
8.5 Threats
8.5.1 Post Recession: Biotech Suffer from Severe Cuts in Capital Funding
8.5.2 The Public are Slow to Accept Novel Technologies
8.5.3 Perception of Risk by the Original Vaccine Manufacturers
9. Conclusion
9.1 Overview
9.2 Regulatory and Quality Standards Create High Barriers of Entry
9.3 Emerging Markets Will Show Stronger Growth
Appendices
Associated Visiongain Reports
Visiongain Report Sales Order Form
Appendix A
About Visiongain
Appendix B
Visiongain report evaluation form
List of Tables
Table 2.1 Timeline of Key Events in Vaccine Development, c.1000 AD – Present
Table 3.1 Vaccine Development Pipeline Using Crucell’s AdVac Technology
Table 3.2 Benefits and Drawbacks of Using Disposable Technology for Vaccine Production
Table 3.3 Companies Providing Disposable Biomanufacturing Platforms/Systems
Table 3.4 Some Leading Pre-Filled Syringe Vaccine Products
Table 3.5 Prominent Lyophilised Vaccines
Table 3.6 Examples of Serum-Free Media for Vaccine Manufacturing
Table 4.1 The Global Vaccine Market Forecast: Revenue ($bn), AGR (%) and CAGR (%) 2017-2028
Table 4.2 The Global Vaccine Market: Revenue ($bn) and Market Share by Submarket (%), 2017-2018
Table 5.1 The Global Vaccine Contract Manufacturing Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.1 The Leading National Vaccine Contract Manufacturing Markets Forecast: Revenue ($m) and Market Share (%), 2017-2018
Table 6.2 The US Vaccine Contract Manufacturing Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.3 The EU5 National Vaccine Contract Manufacturing Markets: Revenue ($m) and Market Share (%) by Country, 2017-2018
Table 6.4 The EU5 Vaccine Contract Manufacturing Market Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.5 The German Vaccine Contract Manufacturing Market Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.6 The Italian Vaccine Contract Manufacturing Market Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.7 The UK Vaccine Contract Manufacturing Market Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.8 The French Vaccine Contract Manufacturing Market Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.9 The Spanish Vaccine Contract Manufacturing Market Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.10 The Japanese Vaccine Contract Manufacturing Market Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.11 The Chinese Vaccine Contract Manufacturing Market Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.12 The Indian Vaccine Contract Manufacturing Market Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.13 The Russian Vaccine Contract Manufacturing Market Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.14 The Brazilian Vaccine Contract Manufacturing Market Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 7.1 Boehringer Ingelheim: Biopharmaceuticals Revenue ($m) and Annual Growth Rate (%), 2010-2016
Table 7.2 Catalent: Revenue($m) and AGR (%), 2010-2016
Table 7.3 Catalent by Segment: Revenue($m), AGR (%), and Intersegment Revenue Eliminations, 2011-2016
Table 7.4 Charles River Laboratories: Revenue ($bn) and AGR (%), 2010-2015
Table 7.5 Charles River Laboratories: Newly Revised Reportable Segments
Table 7.6 Charles River Laboratories: cGMP Vaccine Manufacturing and Testing Capabilities
Table 7.7 Lonza Custom Manufacturing: Revenue ($m) and AGR (%), 2010-2016
Table 7.8 Meridian Bioscience: Revenue ($m), AGR (%), 2010-2016
Table 7.9 Meridian Bioscience: Revenue ($m) and AGR (%) by Segment, 2011-2016
Table 7.10 SynCo Bio Partners Capabilities
Table 8.1 SWOT Analysis of the Vaccine Contract Manufacturing Market
List of Figures
Figure 3.1 Influenza Vaccine Production Using a Cell-Based Manufacturing Method
Figure 3.2 Medicago’s Product Pipeline
Figure 3.3 Novavax’ Insect Egg-Based Vaccine Production Process
Figure 3.4 Pre-filled Syringes Market: Drivers and Restraints, 2018-2028
Figure 4.1 The Global Vaccine Market Forecast: Revenue ($bn), AGR (%) 2017-2028
Figure 4.2 The Global Vaccine Market: Market Share by Submarket (%), 2017
Figure 5.1 Selected Tasks Outsourced in the Pharmaceutical and Biotech Industry
Figure 5.2 The Global Vaccine Contract Manufacturing Forecast: Revenue ($m), 2017, 2022 and 2028
Figure 5.3 The Global Vaccine Contract Manufacturing Forecast: Revenue ($m) and AGR (%), 2017-2028
Figure 6.1 The Leading National Vaccine Contract Manufacturing Markets: Market Share (%) by Region, 2017
Figure 6.2 The US Vaccine Contract Manufacturing Forecast: Revenue ($m) and AGR (%), 2017-2028
Figure 6.3 The EU5 Vaccine Contract Manufacturing Markets: Market Share (%) by Country, 2017
Figure 6.4 The EU5 Vaccine Contract Manufacturing Market Forecast: Revenue ($m) and AGR (%), 2017-2028
Figure 6.5 The EU5 Vaccine Contract Manufacturing Markets: Market Share (%) by Country, 2018
Figure 6.6 The German Vaccine Contract Manufacturing Market Forecast: Revenue ($m) and AGR (%), 2017-2028
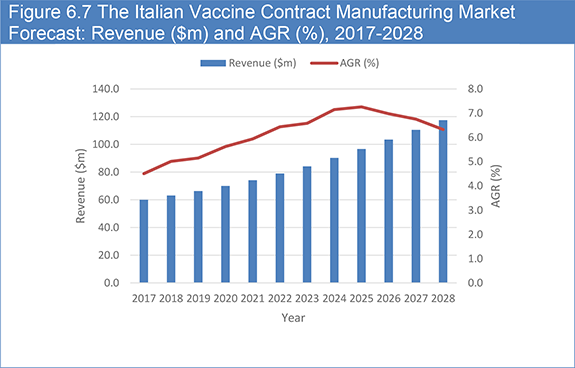
Figure 6.7 The Italian Vaccine Contract Manufacturing Market Forecast: Revenue ($m) and AGR (%), 2017-2028
Figure 6.8 The UK Vaccine Contract Manufacturing Market Forecast: Revenue ($m) and AGR (%), 2017-2028
Figure 6.9 The French Vaccine Contract Manufacturing Market Forecast: Revenue ($m) and AGR (%), 2017-2028
Figure 6.10 The Spanish Vaccine Contract Manufacturing Market Forecast: Revenue ($m) and AGR (%), 2017-2028
Figure 6.11 The Japanese Vaccine Contract Manufacturing Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Figure 6.12 The Chinese Vaccine Contract Manufacturing Market Forecast: Revenue ($m) and AGR (%) 2017-2028
Figure 6.13 The Indian Vaccine Contract Manufacturing Market Forecast: Revenue ($m) and AGR (%) 2017-2028
Figure 6.14 The Russian Vaccine Contract Manufacturing Market Forecast: Revenue ($m) and AGR (%), 2017-2028
Figure 6.15 The Brazilian Vaccine Contract Manufacturing Market Forecast: Revenue ($m) and AGR (%), 2017-2028
Figure 7.1 Boehringer Ingelheim: Biopharmaceuticals Revenue ($m) and Annual Growth Rate (%), 2010-2016
Figure 7.2 Catalent: Revenue ($m) and AGR (%), 2010-2016
Figure 7.3 Catalent: Revenue ($m) by Segment, 2011-2016
Figure 7.4 Catalent: Market Shares (%) by Segment, 2016
Figure 7.5 Charles River Laboratories: Revenue ($bn) and AGR (%), 2010-2016
Figure 7.6 Lonza Custom Manufacturing: Revenue ($m) and AGR (%), 2010-2016
Figure 7.7 Meridian Bioscience: Revenue ($m), and AGR (%), 2010-2016
Figure 7.8 Meridian Bioscience: Revenue ($m) by Segment, 2011-2016
Figure 7.9 Meridian Bioscience: Market Share (%) by Segment,2016
Figure 8.1 Drivers and Restraints for the Vaccine Contract Manufacturing Sector
Accugenix
Aeras Global
AfriVax
Amgen
AmProtein
Antigenics Inc.
Artelis
AstraZeneca
ATM Life Sciences
ATMI
AVEO Pharmaceuticals
Bacteriological Institute of the Anhaltian Administrative Areas
Batavia Bioservices
Baxter
Baxter BioPharma Solutions
Bayer
Beijing Tiantan Biological Productions
Ben Venue Laboratories
Berna Biotech
Bharat Biotech International Limited
Binnopharm
BioKangtai
Biovest
Boehringer Ingelheim
Boehringer Ingelheim Biopharmaceuticals (China)
Boehringer Ingelheim BioXcellence
Cadila Pharmaceuticals
Catalent
Cellexus Limited
Charles River Laboratories
Chengdu Institute of Biological Products Co.
China National Biotec Group Co., Ltd. (CNBG)
Crucell
CSL Biotherapies
Daiichi Sankyo Co. Ltd.
Dalian Hissen Bio-Pharm
Dendreon
Elanco Animal Health
Eli Lilly
Farmindustria
FiberCell
Gallant Custom Laboratories
GE Healthcare
GE Healthcare Life Sciences
GE Wave Biotech
Gelita
GlaxoSmithKline (GSK)
GlaxoSmithKline Biologics
Hikma
Hualan Biological Bacterin
Hyclone
IDT Biologika GmbH
Impfstoffwerk Dessau-Tornau GmbH (IDT)
Institut Pasteur
Intercell Corporation
Invitrogen
Irvine Scientific
Klocke Group
Kuhner
LG Life Sciences
Lonza
Medicago
Medimmune
Meissner Filtration
Merck & Co.
Merck KGaA
Merck Millipore
Merck Serono
Meridian Bioscience
Meridian Life Science
MGC Pharma Co.
Micron Technologies
Millipore
Mitsubishi Tanabe Pharma (MTPC)
MorphoSys
MP Biomedicals
Novartis
Novartis Vaccines and Diagnostics
Novavax
Organon Teknika Corporation
Pall Life Sciences
Paragon Bioservices
Patheon
PaxVax Corporation
PBS Biotech
Pfenex
Pfizer
Pharmaceutical Base Development Co., Ltd.
Philip Morris
Philip Morris Investments
Pierre-Guerin Biolafitte
Protein Sciences Corporation
Refine Technology
Sanofi
Sanofi Pasteur
Sanofi Pasteur MSD
Sartorius Stedim
Shanghai Zhangjiang Biotech
ShangPharma Corporation
Sigma Aldrich
Sigma-Aldrich Fine Chemicals (SAFC)
Sunrise Farms, Inc.
SynCo Bio Partners (SynCo)
Thermo Fisher
Vector Solutions
Vivalis
Wave Biotech
Wyeth
Xcellerex
Xencor
ZellWerk
Zhejiang Jiang Yuan Tang Biotechnology Corporation
List of Organisations Mentioned in the Report
Biomedical Advanced Research and Development Authority (BARDA)
Center for Disease Control (CDC)
China Food and Drug Administration (CFDA)
Defense Advanced Research Projects Agency (DARPA)
Department of Health and Human Services (HHS)
Emory University
European Medical Agency
Food and Drug Administration (FDA)
National Institute of Allergy and Infectious Diseases (NIAID)
PATH
U.S. Department of Health and Human Services Office
U.S. Department of Homeland Security
World Health Organisation (WHO)
Download sample pages
Complete the form below to download your free sample pages for Global Vaccine Contract Manufacturing Market Report 2018-2028
Related reports
-

Global Biosimilars and Follow-On Biologics Market 2018-2028
The global biosimilars and follow-on biologics market is estimated to have reached $7.70bn in 2017 and expected to grow at...
Full DetailsPublished: 01 June 2018 -

Global Pre-Filled Syringes Market Forecast 2019-2029
The global pre-filled syringes market was valued at $9.8bn in 2018. This market is estimated to grow at a CAGR...
Full DetailsPublished: 31 January 2019 -

Global Allergic Rhinitis Drugs Market 2018-2028
The global allergic rhinitis drugs market is expected to grow at a CAGR of 3.3% in the first half of...
Full DetailsPublished: 24 August 2018 -

Pharma Leader Series: Top 50 Pharmaceutical Contract Manufacturing Organisations (CMOs) Market 2018
Contract manufacturing represents the largest sector of the pharma outsourcing industry. Pharmaceutical companies have sought to take advantage of the...Full DetailsPublished: 28 September 2018 -

Biologics Market Trends and Forecasts 2018-2028
The global biologics market is estimated to reach $250bn in 2023. The market is expected to grow at a CAGR...
Full DetailsPublished: 14 November 2018 -

Global Biosimilar Monoclonal Antibodies Forecast 2018-2028
The global biosimilar monoclonal antibodies market is expected to reach $5.9bn in 2023 and is estimated to grow at a...
Full DetailsPublished: 27 February 2018 -

Global Influenza Vaccines Market Outlook 2018-2028
The latest report from business intelligence provider visiongain offers comprehensive analysis of the global influenza vaccines market. Visiongain assesses that...
Full DetailsPublished: 19 June 2018 -

Gene Therapy R&D and Revenue Forecasts 2018-2028
The gene therapy market is projected to grow at a CAGR of 41.1% in the first half of the forecast...
Full DetailsPublished: 31 May 2018 -

Pharmaceutical Contract Manufacturing Market 2018-2028
The pharmaceutical contract manufacturing market is expected to grow at a CAGR of 6.0% in the first half of the...
Full DetailsPublished: 27 June 2018 -

Top 25 Antibiotic Drugs Manufacturers 2018
Visiongain forecasts the antibiotic drugs market to increase to $ 43,841.7m in 2022. The market is estimated to grow at...
Full DetailsPublished: 12 June 2018
Download sample pages
Complete the form below to download your free sample pages for Global Vaccine Contract Manufacturing Market Report 2018-2028
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Latest Pharma news
Visiongain Publishes Drug Delivery Technologies Market Report 2024-2034
The global Drug Delivery Technologies market is estimated at US$1,729.6 billion in 2024 and is projected to grow at a CAGR of 5.5% during the forecast period 2024-2034.
23 April 2024
Visiongain Publishes Cell Therapy Technologies Market Report 2024-2034
The cell therapy technologies market is estimated at US$7,041.3 million in 2024 and is projected to grow at a CAGR of 10.7% during the forecast period 2024-2034.
18 April 2024
Visiongain Publishes Automation in Biopharma Industry Market Report 2024-2034
The global Automation in Biopharma Industry market is estimated at US$1,954.3 million in 2024 and is projected to grow at a CAGR of 7% during the forecast period 2024-2034.
17 April 2024
Visiongain Publishes Anti-obesity Drugs Market Report 2024-2034
The global Anti-obesity Drugs market is estimated at US$11,540.2 million in 2024 and is expected to register a CAGR of 21.2% from 2024 to 2034.
12 April 2024

















