Industries > Pharma > Gene Therapy R&D and Revenue Forecasts 2018-2028
Gene Therapy R&D and Revenue Forecasts 2018-2028
Prospects for Drugs Treating Cancer, Cardiovascular Disorders, Rare Diseases, Ophthalmologic Conditions, Other Diseases, Approved Gene therapies, R&D Pipeline Analysis, Gene Therapy Regulations by Region and Leading Players
The gene therapy market is projected to grow at a CAGR of 41.1% in the first half of the forecast period. In 2018, the cancer treatment submarket accounted for 68.7% of the gene therapy drug market. Visiongain estimated that gene therapy for rare diseases will be the driver for market growth in the second half of the forecast period.
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in this sector.
In this brand-new 134 page report you will receive 72 charts– all unavailable elsewhere.
The 134-page report provides clear detailed insight into the gene therapy market. Discover the key drivers and challenges affecting the market.
By ordering and reading our brand-new report today you stay better informed and ready to act.
Report Scope
• Gene Therapy market forecasts from 2018-2028
• This report assesses the approved gene therapy products in the market and gives revenue to 2028 for Neovasculgen
• Provides qualitative analysis and forecast of the submarket by indication for the period 2018-2028:
• Cancer
• Cardiovascular disorders
• Rare diseases
• Ophthalmological diseases
• Other therapeutic uses
• Profiles leading companies that will be important in the development of the gene therapy market. For each company, developments and outlooks are discussed and companies covered in this chapter include:
• UniQure
• Biogen
• Bluebird Bio
• Spark Therapeutics
• Applied Genetics Technologies Corporation
• Oxford Biomedica
• GenSight Biologics
• Assesses the outlook for the leading gene treatment R&D pipeline for 2018 and discusses technological progress and potential. Profiles appear for gene therapy drug candidates, with revenue forecasts for six leading agents:
• SPK-RPE65 (Spark Therapeutics)
• Collategene (AMG0001, AnGes MG/Vical)
• Invossa (TissueGene-C, TissueGene Inc/Kolon Life Science)
• BC-819 (BioCancell)
• Lenti-D (Bluebird Bio)
• GSK2696273 (GlaxoSmithKline)
• Provides qualitative analysis of trends that will affect the gene therapies market, from the perspective of pharmaceutical companies, during the period 2018 to 2028. SWOT analysis is provided and an overview of regulation of the gene therapy market by leading region given.
• Our study discusses factors that influence the market including these:
• Translation of research into marketable products modifying human DNA – gene transfer for therapeutic use, altering the nuclear genome
• Genomic editing technology and other supporting components
• Collaborations to develop and launch gene-based products – acquisitions and licensing deals
• Supporting technologies for human genetic modification, gene replacement and targeted drug delivery
• Gene therapies for ophthalmologic diseases – next-generation medicines
• Regulations in the United States, the European Union and Japan – overcoming technological and medical challenges to pass clinical trials.
Visiongain’s study is intended for anyone requiring commercial analyses for the gene therapy market. You find data, trends and predictions.
Buy our report today Gene Therapy R&D Market Research: Prospects for Drugs Treating Cancer, Cardiovascular Disorders, Rare Diseases, Ophthalmologic Conditions, Other Diseases, Approved Gene therapies, R&D Pipeline Analysis, Gene Therapy Regulations by Region and Leading Players.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
1. Report Overview
1.1 Global Gene Therapy Market Overview
1.2 Benefits of This Report
1.3 How This Report Delivers
1.4 Key Questions Answered by This Analytical Report
1.5 Who is This Report For?
1.6 Methods of the Study
1.7 Frequently Asked Questions (FAQ)
1.8 Associated Visiongain Reports
1.9 About Visiongain
2. Introduction to Gene Therapy
2.1 What is Gene Therapy?
2.2 Types of Gene Therapy
2.3 Products Excluded from the Report
2.4 Gene Therapy Vectors
3. Gene Therapy World Market: Leading Treatments and Forecasts, 2017-2028
3.1 The World Market for Gene Therapy: Overview of Products
3.2 Gendicine (rAd-p53) – The First Gene Therapy to Market
3.3 Oncorine: Growth is Hindered by Certain Factors
3.4 Neovasculgen: Russia’s First Gene Therapy
3.4.1 Neovasculgen: Historical Performance and Revenue Forecast, 2017-2028
3.5 Glybera: A Drug with Promise
3.5.1 Glybera: Pricing Ends the Dream to Become a Blockbuster Product
3.6 Strimvelis: Second Gene Therapy for an Inherited Disease Ever to be approved for Sale and the First Corrective Gene Therapy for Children to be Approved Anywhere in the World
3.6.1 Even Big Pharma Struggle in the Rare Disease Space
4. Gene Therapy World Market and Disease Submarkets, 2017-2028
4.1 The Global Market for Gene Therapy Drug Treatments in 2017
4.2 Gene Therapy Market Forecast, 2017-2028
4.3 Gene Therapy for Cancer
4.3.1 Clinical Trials for Cancer
4.3.2 The Cancer Gene Therapy Treatment Submarket Forecast 2017-2028
4.4 Gene Therapy for Rare Diseases
4.4.1 The Rare Disease Treatment Submarket Forecast 2017-2028
4.5 Gene Therapy for Cardiovascular Diseases
4.5.1 The Cardiovascular Disease Treatment Submarket Forecast 2017-2028
4.6 Gene Therapy for Ophthalmologic Diseases
4.6.1 Advanced Stage Gene Therapies for Ophthalmologic Diseases
4.6.2 The Ophthalmologic Disease Treatment Submarket Forecast 2017-2028
4.7 Gene Therapy for Other Diseases
4.7.1 The Other Disease Treatment Submarket Forecast 2017-2028
4.7.2 Changing Market Shares: Cancer Disease Gene Therapies Will Dominate and Become Market Leader
5. R&D Pipeline Analysis and Forecasts, 2017-2028
5.1 Pipeline
5.2 Pipeline Analysis
5.3 Collategene (AMG0001) - AnGes MG/Vical
5.4 BC-819 - BioCancell
5.5 BC-821 - BioCancell
5.6 Lenti-D - Bluebird Bio
5.7 LentiGlobin (LentiGlobin BB305) - Bluebird Bio
5.8 SPK-RPE65 - Spark Therapeutics
5.9 SPK-CHM - Spark Therapeutics
5.10 SPK-FIX - Spark Therapeutics/Pfizer
5.11 SPK-TPP1- Spark Therapeutics
5.12 Invossa (TissueGene-C) - TissueGene Inc/Kolon Life Science
5.13 VM202-DPN - ViroMed
5.14 Important Pipeline Technology Developments
5.15 Clustered regularly interspaced short palindromic repeats (CRISPR)
5.16 The Future of the Gene Therapy Pipeline
6. Companies of Interest in the Gene Therapy Market: Robust Pipelines Will Yield Revenue in the Future
6.1 UniQure- the First Commercial Stage Gene Therapy Company in the West
6.2 Biogen - Focusing on Ophthalmologic and Rare Disease Gene Therapies
6.3 Bluebird Bio: Gene Editing Technology
6.4 Spark Therapeutics- Strong Change to Become the First Company with Marketed Gene Therapy in the US
6.5 Applied Genetic Technologies Corporation: Specialisation in Ophthalmologic Diseases
6.6 Oxford Biomedica: Collaboration as a Strategy
6.7 GenSight Biologics - Novel Mitochondrial Technology Platform
6.8 Gene Therapy and Big Pharma: Collaborations
7. Qualitative Analysis of the Gene Therapy Market, 2018-2028
7.1 SWOT Analysis of the Gene Therapy Treatment Market, 2018
7.2 Strengths: A Vast Pipeline for a Wide Range of Indications
7.3 Weaknesses: Low Patient Populations Cannot Sustain Long-Term Growth
7.4 Opportunities and Threats for the Gene Therapy Drug Market, 2018-2028
7.5 Opportunities: Collaborations and Novel Technologies Will Pave the Way
7.6 Threats: Expensive Drugs with Strict Regulations
7.7 National Markets: Regulation of the Gene Therapy Market
7.7.1 Gene Therapy Regulations: Evolving with the Market
7.7.2 US Regulations: Strict Regulation of a Cautious Market
7.7.3 EU Regulations: One Approval So Far
7.7.4 Japanese Regulations: A Rigorous Process
7.7.5 China Has Highest Gene Sequencing Capacity
8. Conclusions
8.1 The World Gene Therapy Treatment Market: Current World Outlook
8.1.1 Leading Gene Therapy Treatment Submarkets and Products, 2017-2028
8.1.2 Driver for Growth to 2028: A Vast Pipeline of Gene Therapy Products
8.1.3 Leading Regional Markets and Companies: Partnerships Will Achieve Success
8.2 The Future of the Gene Therapy Treatment Market
Associated Visiongain Reports
Visiongain report sales order form
About Visiongain
Visiongain Report Evaluation Form
Table of Tables
Table 1.1 Foreign Currency Exchange Rates Utilised: Exchange Rate to $US, 2015, 2016, and 2017
Table 3.1 Approved Gene Therapies, 2017
Table 3.2 Gene Therapies in Pipeline, 2017
Table 3.3 Neovasculgen Historical Revenue Comparisons, Revenue ($m), AGR (%), 2013-2017
Table 3.4 Neovasculgen Forecast, Revenue ($m), AGR (%), CAGR (%), 2017-2028
Table 4.1 Leading Gene Therapy Drugs by Revenue ($m), 2017
Table 4.2 World Market Forecasts for Gene Therapy Drugs by Indication, Market Sizes ($m), Annual Growth Rates (%), CAGR (%), 2017-2028
Table 4.3 Selected Anti-Angiogenic Gene Therapy Using a Non-Viral Vector, 2017
Table 4.4 Selected Anti-Angiogenic Gene Therapy Using a Retroviral Vector, 2017
Table 4.5 Selected Anti-Angiogenic Gene Therapy Using an Adenoviral Vector, 2017
Table 4.6 Selected Anti-Angiogenic Gene Therapy Using an Adeno-Associated Virus Vector, 2017
Table 4.7 Selected Anti-Angiogenic Gene Therapy Using Lentiviral Vector, 2017
Table 4.8 Global Gene Therapy Clinical Trials by Indication, 2017
Table 4.9 Selected Cancer Gene Therapies in Phase 2 and Phase 3 Clinical Trial Development, 2017
Table 4.10 Selected Cancer Gene Therapies in Phase 1 and Phase 1/2 Clinical Trial Development, 2017
Table 4.11 Global Gene Therapy Market: Revenue ($m) and Market Shares (%) by Indication, 2017
Table 4.12 Global Market Forecast for Cancer Gene Therapies: Revenue ($m), Annual Growth Rate (%), CAGR (%), 2017-2028
Table 4.13 Rare Diseases Gene Therapy Pipeline, 2017
Table 4.14 Global Market Forecast for Rare Disease Gene Therapies: Revenue ($m), Annual Growth Rate (%), CAGR (%), 2017-2028
Table 4.15 Cardiovascular Gene Therapy Pipeline, 2017
Table 4.16 Global Market Forecast for Cardiovascular Disease Gene Therapies: Revenue ($m), Annual Growth Rate (%), CAGR (%), 2017-2028
Table 4.17 Preclinical Ophthalmologic Pipeline, 2017
Table 4.18 Clinical Ophthalmologic Pipeline, 2017
Table 4.19 Additional Products in the Clinical Ophthalmologic Pipeline, 2017
Table 4.20 Global Market Forecast for Ophthalmologic Disease Gene Therapies: Revenue ($m), Annual Growth Rate (%), CAGR (%), 2017-2028
Table 4.21 Global Market Forecast for Other Disease Gene Therapies: Revenue ($m), Annual Growth Rate (%), CAGR (%), 2017-2028
Table 4.22 Global Market Forecast for Gene Therapies by Indication: Market Shares (%), 2017-2022
Table 4.23 Global Market Forecast for Gene Therapies by Indication: Market Shares (%), 2023-2028
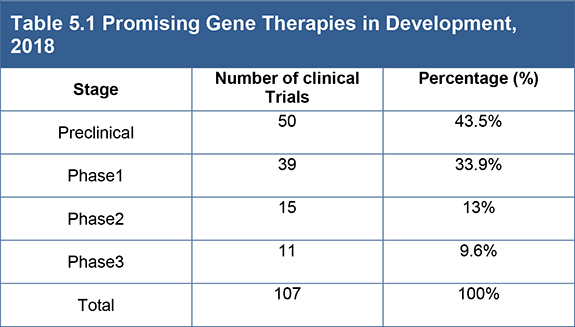
Table 5.1 Promising Gene Therapies in Development, 2018
Table 5.2 Gene Therapies in Phase 3, 2017
Table 5.3 Collatagene Forecast, Revenue ($m), AGR (%), CAGR (%), 2017-2028
Table 5.4 BC-819 Forecast, Revenue ($m), AGR (%), CAGR (%), 2017-2028
Table5.5 Lenti-D Forecast, Revenue ($m), AGR (%), CAGR (%), 2017-2028
Table 5.6 SPK-RPE65 Forecast, Revenue ($m), AGR (%), CAGR (%), 2017-2028
Table 5.7 Invossa Forecast, Revenue ($m), AGR (%), CAGR (%), 2017-2028
Table 6.1 uniQure Pipeline, 2017
Table 6.2 Spark Therapeutics Collaborations, 2017
Table 6.3 Selected Big Pharma Collaborations in Gene Therapy, 2017
Table 7.1 Strengths and Weaknesses of the Gene Therapy Drug Treatment Market, 2017
Table 7.2 Gene Therapy Pipeline for Neurodegenerative Diseases, 2017
Table 7.3 Gene Therapy Pipeline for Metabolic Diseases, 2017
Table 7.4 Gene Therapy Pipeline for Musculoskeletal Diseases, 2017
Table 7.5 Gene Therapy Pipeline for Viral Diseases, 2017
Table 7.6 Opportunities and Threats of the Gene Therapy Drug Market, 2017
Table 7.7 Clinical Trials for Standard Drug Development Versus Orphan Drug Development, 2017
Table 8.1 Global Gene Therapy Drug Market Forecasts by Indication: Market Size ($m), Market Share (%), CAGR (%), 2017, 2022 and 2028
Table of Figures
Figure 1.1 Global Gene Therapy Market Segmentation Overview, 2017
Figure 2.1 Vectors Used in Gene Therapy Clinical Trials, 2017
Figure 3.1 Neovasculgen Forecast, Revenue ($m), 2017-2028
Figure 4.1 Gene Therapy World Market Forecast, Market Size ($m), AGR (%), 2017-2028
Figure 4.2 Global Gene Therapy Clinical Trials by Indication, 2017
Figure 4.3 Cancer Gene Therapy Pipeline by Phase of Development, 2017
Figure 4.4 Global Gene Therapy Market: Market Shares (%) by Indication, 2018
Figure 4.5 Global Market Forecast for Cancer Gene Therapies: Revenue ($m), 2017-2028
Figure 4.6 Rare Diseases Gene Therapy Pipeline by Phase of Development, 2017
Figure 4.7 Global Market Forecast for Rare Disease Gene Therapies: Revenue ($m), 2017-2028
Figure 4.8 Cardiovascular Gene Therapy Pipeline by Phase of Development, 2017
Figure 4.9 Global Market Forecast for Cardiovascular Disease Gene Therapies: Revenue ($m), 2017-2028
Figure 4.10 Global Market Forecast for Ophthalmologic Disease Gene Therapies: Revenue ($m), 2017-2028
Figure 4.11 Global Market Forecast for Other Disease Gene Therapies: Revenue ($m), 2017-2028
Figure 4.12 Global Market for Gene Therapy Drugs: Market Shares by Indication (%), 2022
Figure 4.13 Global Market for Gene Therapy Drugs: Market Shares by Indication (%), 2028
Figure 5.1 Gene Therapies in Clinical Development by Phase, 2017
Figure 5.2 Collategene Forecast, Revenue ($m), 2017-2028
Figure 5.3 BC-819 Forecast, Revenue ($m), 2017-2028
Figure 5.4 Lenti-D Forecast, Revenue ($m), 2017-2028
Figure 5.5 SPK-RPE65 Forecast, Revenue ($m), 2017-2028
Figure 5.6 Invossa Forecast, Revenue ($m), 2017-2028
Figure 5.7 Gene Therapy Market Strategy Analysis
Figure 5.8 Key Take Ways, Challenges and Application of CRISPR Market
Figure 5.9 Impact of CRISPR Technology on the Gene Therapy Market
Figure 8.1 Gene Therapy Drug Treatment Market Forecast by Indication: Market Sizes ($m), 2017, 2022, and 2028
4DMT (4D Molecular Therapeutics)
Abeona
AGTC (Applied Genetics Technologies Corporation)
AMT (Amsterdam Molecular Therapeutics)
AnGes MG
Asklepios BioPharma
AstraZeneca
Audentes Therapeutics
Avalanche Biotech
Bayer Healthcare
Beijing Northland Biotech Co
Benda Pharmaceutical
Benitec Biopharma
BioCancell
Biogen
Biogen Idec
Bluebird Bio
BMS (Bristol-Myers Squibb)
Broad Institute/Whitehead Institute
Celgene
Cell Therapy Catapult
Cellectis
Chiesi Farmaceutici
Clearside Biomedical
Convergence Pharmaceuticals
Daiichi Sankyo
Dimension Therapeutics
Editas Medicine
Fondazione Telethon
Francis Crick Institute
Genable Technologies Ltd
Genethon
GenSight Biologics
GenVec
Google
GSK (GlaxoSmithKline)
Henry Ford Health System
HSCI (Human Stem Cells Institute)
HSR-TIGET (San Raffaele Telethon Institute for Gene Therapy),
ImaginAb
Immune Design Corp
InoCard
Inovio
Intellia Therapeutics
Invetech
Kite Pharma
Kolon Group
Kolon Life Science
Lysogene
Mitsubishi Tanabe Pharma Corporation
Neuralgene
NightstaRx
Northwestern Memorial Hospital
Novartis
OXB (Oxford Biomedica)
Pfizer
PNP Therapeutics
Precision Genome Engineering Inc aka Pregenen
ProNai
Protek Group
Raffaele Hospital
REGENX Biosciences
Renova Therapeutics
Roche
Roszdravnadzor
Sangamo Biosciences
Sanofi
Sarepta Therapeutics
Shanghai Sunway Biotech
Shenzhen SiBiono GeneTech
Sotex Pharm Firm
Spark Therapeutics
SynerGene Therapeutics
Takara Bio
TAP Biosystems
Thermo Fisher Scientific
TissueGene
ToolGen
UC Berkeley
UC San Francisco
uniQure
US Business Innovation Network
Vertex Pharmaceuticals
Vical Incorporated
ViroMed
VM Biopharma
Voyage Therapeutics
List of Organisation Mentioned
ASCO (American Society of Clinical Oncology)
ASI (Agency for Strategic Initiatives)
CAT (Committee for Advanced Therapies)
CBER (Center for Biologics Evaluation and Research)
CHMP (Committee for Medicinal Products for Human Use)
CHOP (The Children’s Hospital of Philadelphia)
DCGI (Drugs Controller General of India)
DHHS (Department of Health and Human Services)
EMA (European Medicines Agency)
FDA (US Food and Drug Administration)
INSERM (Institut National de la Santé et de la Recherche Médicale)
IRB (Institutional Review Boards)
MFDS (Korean Ministry of Food and Drug Safety)
MHLW (Ministry of Health, Labour, and Welfare)
MHRA (Medicines and Healthcare Products Regulatory Agency)
Ministry of Health Commission
NHS (National Health Service)
NICE (the National Institute for Health and Care Excellence)
NIH (National Institutes of Health)
OHRP (Office for Human Research Protections)
PMDA (Pharmaceuticals and Medical Devices Agency)
RCGM (Review Committee of Genetic Manipulation)
Russian Ministry of Healthcare and Social Development
SFDA (State Food and Drug Administration of China)
SMC (Scottish Medicines Consortium)
The Fund for Promotion of Small Innovative Enterprises in Science and Technology
The IGI (Innovative Genomics Initiative)
The Innovative Genomics Initiative
The Walter and Eliza Hall Institute
The Wellcome Trust Sanger Institute
WFH (World Federation of Hemophilia)
WHO (World Health Organization)
Download sample pages
Complete the form below to download your free sample pages for Gene Therapy R&D and Revenue Forecasts 2018-2028
Related reports
-

Generic Drugs Market Forecast 2018-2028
The generic drugs market is estimated at $257.3bn in 2017 and is expected to grow at a CAGR of 7.9%...
Full DetailsPublished: 25 April 2018 -

Checkpoint Inhibitors for Treating Cancer Market Report 2018-2028
Our 161-page report provides 107 tables, charts, and graphs. Read on to discover the most lucrative areas in the industry...Full DetailsPublished: 23 April 2018 -

Global Stem Cell Technologies and Applications Market 2018-2028
The global stem cell technologies and applications market is estimated to have reached $12,040 million and is expected to grow...
Full DetailsPublished: 02 March 2018 -

Global Ophthalmic Drugs Market Forecast 2017-2027
The global ophthalmic drugs market is expected to grow at a CAGR of 4.0% in the first half of the...Full DetailsPublished: 14 June 2017 -

Global Transplant Market Forecast 2019-2029
The global transplant market will reach $29bn in 2019 and is estimated to grow at a CAGR of 9.9% from...
Full DetailsPublished: 18 December 2018 -

Biological Drug API Manufacturing Services World Industry and Market Predictions 2018-2028
The biological drug API manufacturing market is estimated to grow at a CAGR of 9.0% in the first half of...
Full DetailsPublished: 06 July 2018 -

Global Genomics Market Report 2018-2028
The global genomics market is estimated to reach $23bn by 2022. In 2017, the diagnostic test segment held 27% of...Full DetailsPublished: 31 October 2018 -

Global Acute Myeloid Leukaemia Market Forecast 2018-2028
The global acute myeloid leukaemia market reached $1bn in 2017 and is estimated to reach $2.8bn by 2022. In 2017,...
Full DetailsPublished: 21 September 2018 -

Global Macular Degeneration (AMD) and Other Retinal Diseases Drugs Industry and Market 2018-2028
Our 171-page report provides 140 tables, charts, and graphs. Read on to discover the most lucrative areas in the industry...
Full DetailsPublished: 30 May 2018 -

Vaccine Sales Market Forecast 2018-2028
The Global Vaccines Sales market was valued at $36.9 billion in 2017. This value will grow to $61.1bn in 2022...
Full DetailsPublished: 09 March 2018
Download sample pages
Complete the form below to download your free sample pages for Gene Therapy R&D and Revenue Forecasts 2018-2028
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Don’t Miss Out!
Subscribe to receive the latest Industry news, trending insight and analysis straight to your inbox.Choose your preferences:
Latest Pharma news
Retinal Gene Therapy Market
The global Retinal Gene Therapy market is projected to grow at a CAGR of 9.6% by 2034
26 July 2024
HIV Drugs and Injectables Market
The global HIV Drugs & Injectables market is projected to grow at a CAGR of 4.6 % by 2034
24 July 2024
Digital Twin Technology in Pharmaceutical Manufacturing Market
The global Digital Twin Technology in Pharmaceutical Manufacturing market is projected to grow at a CAGR of 31.3% by 2034
23 July 2024
Specialty Pharma Market
The global Specialty Pharma market is projected to grow at a CAGR of 7.5% by 2034
22 July 2024