Industries > Pharma > Global Biosimilar Monoclonal Antibodies Forecast 2018-2028
Global Biosimilar Monoclonal Antibodies Forecast 2018-2028
Biosimilar Versions of Infliximab, Rituximab, Trastuzumab, Adalimumab, Bevacizumab and Abciximab
The global biosimilar monoclonal antibodies market is expected to reach $5.9bn in 2023 and is estimated to grow at a CAGR of 19% from 2016 to 2028.
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in this sector.
In this brand new 204-page report you will receive 72 tables and 74 figures– all unavailable elsewhere.
The 204-page report provides clear detailed insight into the global biosimilar monoclonal antibodies market. Discover the key drivers and challenges affecting the market.
By ordering and reading our brand new report today you stay better informed and ready to act.
Report Scope
• Global Biosimilar Monoclonal Antibodies Market forecasts from 2018-2028
• Along with revenue prediction for the overall world market for biosimilar monoclonal antibodies, our investigation shows forecasts to 2028 for the market segmented by compound:
• Infliximab
• Rituximab
• Abciximab
• Trastuzumab
• Adalimumab
• Bevacizumab
• This report also shows revenue to 2028 for individual biosimilar mAb products in the market:
• Remsima/Inflectra
• Infimab
• Reditux
• BI695500
• CT-P10
• BI695501
• FKB327
• FKB238
• Mabtas
• AcellBia
• Maball
• Clotinab
• Abcixirel
• BCD-022
• BCD-021
• Herzuma
• CANMAB/Hertraz
• Our analyses show individual revenue forecasts to 2028 for these regional and national markets:
• The US Biosimilar mAb Market
• Japanese Biosimilar mAb Market
• EU5 Biosimilar mAb Markets
• BRIC and South Korea Biosimilar mAb Markets
• Rest of the World Biosimilar mAb Market
• This report profiles 10 leading companies either with biosimilar mAbs already on the market or in the pipeline
• Our study discusses strengths, weaknesses, opportunities and threats affecting the biosimilar monoclonal antibodies market
Visiongain’s study is intended for anyone requiring commercial analyses for the biosimilars monoclonal antibodies market. You find data, trends and predictions.
Buy our report today Global Biosimilar Monoclonal Antibodies Forecast 2018-2028: Biosimilar Versions of Infliximab, Rituximab, Trastuzumab, Adalimumab, Bevacizumab and Abciximab.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
1. Report Overview
1.1 Overview of Biosimilar Monoclonal Antibodies: R&D, Industry and Market
1.2 Why You Should Read this Report
1.3 How This Study Delivers
1.4 Main Questions Answered by the Analysis
1.5 Who is This Investigation For?
1.6 Method of Research and Analysis
1.7 Frequently Asked Questions (FAQs)
1.8 Associated visiongain Reports
1.9 About visiongain
2. Introduction to Biosimilar Monoclonal Antibodies
2.1 Natural Antibodies: Key to the Immune System
2.2 From Serum therapy to Monoclonal Antibodies
2.3 Humanising the mAb
2.4 Biologics (Biological Drugs) and Biosimilars
2.5 Why are Biosimilars in High Demand?
2.6 Considerations for the Development of Biosimilars
2.6.1 Biologics and Biosimilars Are Large, Complex Molecules
2.6.2 How Much of a Concern is Immunogenicity?
2.7 Biosimilar Monoclonal Antibodies Currently on the Market
3. The Global Market for Biosimilar Monoclonal Antibodies, 2018-2028
3.1 The Present Global Market for Biosimilar Monoclonal Antibodies
3.2 The Global Market for Biosimilar mAbs: A Revenue Forecast, 2016-2028
3.3 The Global Market for Biosimilar mAbs by Compound: Predictions for Revenue and Market Share in 2023 and 2028
3.4 The World Market for Biosimilar mAbs by Compound: Revenue Forecasts, 2016-2028
3.4.1 Will Biosimilar Versions of Monoclonal Antibodies Capture a Large Proportion of the Market?
3.4.2 Biosimilar Infliximab
3.4.3 Biosimilar Rituximab
3.4.4 Biosimilar Abciximab
3.4.5 Biosimilar Trastuzumab
3.4.6 Biosimilar Adalimumab
3.4.7 Biosimilar Bevacizumab
3.5 Drivers and Restraints for the Biosimilar mAb market 2018-2028
3.5.1 Driving Factors of the Biosimilar mAb Market 2018-2028
3.6 Restraining Factors of the Biosimilar mAb Market 2018-2028
4. Individual Biosimilar mAb Products: Marketed and Pipeline Drugs, 2018-2028
4.1 Biosimilar mAbs on the Market in 2017
4.1.1 Celltrion’s Remsima/ Hospira’s Inflectra/ Egis’ Flammegis (Infliximab): The First Official Biosimilar Monoclonal Antibody
4.1.2 Infimab (Infliximab): Launched a Quarter Ahead of Schedule
4.1.3 Dr Reddy’s Reditux (Rituximab): No Application for EU or US Approval
4.1.4 Intas Pharmaceuticals’ Mabtas (Rituximab)
4.1.5 AcellBia (Rituximab): Sales in Russia Generating Large Revenue Figures
4.1.6 MABALL: Faces Competition from other Biosimilar Rituximabs in India
4.1.7 Isu Abxis’ Clotinab (abciximab):
4.1.8 AbcixiRel: The First Indian Abciximab Biosimilar
4.1.9 Herzuma (Trastuzumab): Approved in South Korea
4.1.10 CANMAb/Hertraz (Trastuzumab)
4.2 Pipeline Biosimilars
4.2.1 BI695500 (Rituximab)
4.2.2 CT-P10 (Rituximab): Celltrion’s Reputation for Biosimilars will Assist Sales
4.2.3 Boehringer Ingelheim’s BI695501 (Adalimumab)
4.2.4 BCD-022 (Trastuzumab): Approved in Russia
4.2.5 Fujifilm Kyowa Kirin Biologics’ FKB327 (Adalimumab)
4.2.6 Fujifilm Kyowa Kirin Biologics’ FKB238 (Bevacizumab)
4.2.7 BCD-021 (Bevacizumab): Russia Will Contribute the Most Revenue
5. Leading National Markets for Biosimilar Monoclonal Antibodies, 2018-2028
5.1 What Were the Leading National Markets for Biosimilar mAbs in 2017?
5.2 How Will National Market Shares Change Over the Forecast Period?
5.3 India: The Current Leading Nation for Biosimilar mAbs but Growth Will Slow
5.3.1 India Releases New Biosimilar Development Guidelines
5.3.2 Indian Biosimilar Submarket Forecast 2016-2028
5.4 Russia: Lack of Regulatory Framework Works Well in the Short Term
5.4.1 Russian Government Backed ‘Pharma 2020’ Initiative
5.4.2 Russian Biosimilar mAb Revenue Forecast, 2016-2028
5.4 South Korean Market Has Benefitted from Early Biosimilar Guidelines
5.4.1 Significant Investment in Biosimilars in South Korea
5.4.2 South Korean Biosimilar mAb Revenue Forecast, 2016-2028
5.5 China: A Fragmented Biosimilar mAb Market
5.5.1 Chinese FDA Publishes Finalised Biosimilar Guidelines in 2017
5.5.2 China Biosimilar mAb Revenue Forecast, 2016-2028
5.6 Brazil: ANVISA’s Biosimilar Regulations Are Similar the EMA’s
5.6.1 Brazil Biosimilar mAb Revenue Forecast 2016-2028
5.7 The Outlook for Biosimilar mAbs in the EU 2016-2028
5.7.1 Updating Biosimilar Regulation: Guidelines for the Specific Approval of Biosimilar mAbs
5.7.2 Biosimilar mAbs in the EU5: Revenue Forecasts, 2016-2028
5.7.3 Germany: The Current EU Leader
5.7.4 France: Will the Uptake of Biosimilar mAbs be Restricted?
5.7.5 UK: NICE Recommends Use of Remsima for Certain Indications
5.7.6 Italy: Healthcare Spending Cuts Will Drive Growth
5.7.7 Spain: High Biosimilar mAb Discounts Expected
5.8 The Outlook for Biosimilar mAbs in Japan
5.8.1 Regulations for Naming Biosimilars
5.8.2 Biosimilar mAb Market Activity in Japan
5.8.3 Japanese Biosimilar Submarket Forecast 2016-2028
5.9 US Biosimilar mAb Outlook: None Currently on the Market
5.9.1 US FDA Biosimilar Guidelines were Released in 2015
5.9.2 Legal Challenges for Biosimilars in the US
5.9.3 State Regulation of Biosimilar Substitution
5.9.4 The US Biosimilar mAb Market Revenue Forecast, 2016-2028
5.9.5 The Mexico Biosimilar mAb Market Revenue Forecast, 2016-2028
5.9.6 The Canada Biosimilar mAb Market Revenue Forecast, 2016-2028
6. Leading Companies in the Biosimilar Monoclonal Antibodies Market
6.1 Collaboration in Biosimilar mAb Development
6.2 BioXpress
6.3 Celltrion
6.4 Harvest Moon
6.5 Genor Biopharma
6.6 Samsung Bioepsis
6.7 Mabion
6.8 Gene Techno Science
6.9 India’s Biocon
6.10 Coherus Biosciences
6.11 BIOCAD
6.12 Big Pharma and the Biosimilar mAb Market
7. Qualitative Analysis of Biosimilar Monoclonal Antibodies Market
7.1 Strengths and Weaknesses of the Biosimilar Monoclonal Antibodies Market, 2016-2028
7.1.1 Global Demand for Affordable Biopharmaceuticals has Never Been Greater
7.1.2 Adoption of Biosimilar mAbs will be Proportional to the Rate of Discount
7.1.3 Approval Pathways are Now Established in Developed Markets
7.1.4 Complexity of Protein Molecules Leads to Technical Challenges
7.1.5 Doctor and Patient Confidence May Take Time
7.2 Opportunities and Threat Facing the Biosimilar Monoclonal Antibodies Market, 2016-2028
7.2.1 Market Opportunities from Patent Expiries and a Well-Stocked Pipeline
7.2.2 Substitution may Not Occur Automatically
7.2.3 Biobetters Offer a Serious Threat to the Production and Uptake of Biosimilars
7.3 Social and Technological Forces Influencing the Biosimilar Monoclonal Antibodies Market 2016-2028
7.3.1 Social Factors: Driving or Restraining the Market?
7.3.2 Technological Factors Concentrate on Reducing Costs and Increasing Ease of Production
7.3.3 Economic Pressures Raise Demand for Biosimilar mAbs
7.3.4 Political Issues: Stringent Regulations Hurdles to Market Entry
8. Conclusions
8.1 Biosimilar Monoclonal Antibodies: World Market, 2016-2028
8.2 Future of Compounds on the Market and in the Pipeline
8.3 Leading National Markets for Biosimilar Monoclonal Antibodies 2017, 2023, 2028
8.4 Leading Companies in the Biosimilar mAb Market
8.5 Current and Expected Industry Trends 2016-2028
8.5.1 Cost Control of Healthcare Will Work in Favour mAbs
8.5.2 Upcoming Patent Expiries in Developed Markets Offer Large Market Potential
8.5.3 Complex Production Processes are a Restraining Factor
8.5.4 Clinician and Patient Confidence Must be Maximized to Ensure Uptake
8.5.5 Biobetters Pose a Threat
Appendices
Associated Visiongain Reports
Visiongain Report Sales Order Form
Appendix A
About Visiongain
Appendix B
Visiongain report evaluation form
List of Tables
Table 2.1 Classification of Monoclonal Antibodies
Table 2.2 Murine Monoclonal Antibodies on the Market
Table 2.3 Chimeric Monoclonal Antibodies on the Market
Table 2.4 Humanised Monoclonal Antibodies on the Market
Table 2.5 Fully Human Monoclonal Antibodies on the Market
Table 2.6 Revenue of Originator Monoclonal Antibody Products in 2016
Table 3.1 Global Biosimilar mAbs Market Forecast 2016-2028: Revenue ($m), AGR (%), CAGR (%)
Table 3.2 Global Market for Biosimilar mAbs: Revenues ($m) and Market Shares (%) by Reference Compound, 2017, 2023 and 2028
Table 3.3 EU and US Patent Expiry Dates for the Main mAb Reference Products
Table 3.4 Global Biosimilar mAbs Market Forecast by Reference Compound: Revenue ($m), AGR (%), CAGR (%), 2016-2028
Table 3.5 Biosimilar Infliximab: Compounds Awaiting Approval in the Pipeline
Table 3.6 Global Biosimilar Infliximab Market Forecast 2016-2028: Revenue ($m), AGR (%) and CAGR (%)
Table 3.7 Biosimilar Rituximab: Compounds (Marketed or in the Pipeline), 2018
Table 3.8 Global Biosimilar Rituximab Market Forecast 2016-2028: Revenue ($m), AGR (%) and CAGR (%)
Table 3.9 Biosimilar Abciximab: Compounds (Marketed or in the Pipeline), 2018
Table 3.10 Global Biosimilar Abciximab Market Forecast 2016-2028: Revenue ($m), AGR (%) and CAGR (%)
Table 3.11 Biosimilar Trastuzumab: Compounds Marketed and in the Pipeline, 2018
Table 3.12 Global Biosimilar Trastuzumab Market Forecast 2016-2028: Revenue ($m), AGR (%) and CAGR (%)
Table 3.13 Biosimilar Adalimumab: Compounds (Marketed or in the Pipeline) 2018
Table 3.14 Global Biosimilar Adalimumab Market Forecast 2016-2028: Revenue ($m), AGR (%), and CAGR (%)
Table 3.15 Biosimilar Bevacizumab: Compounds (Marketed or in the Pipeline) 2018
Table 3.16 Global Biosimilar Bevacizumab Market Forecast 2016-2028: Revenue ($m), AGR (%) and CAGR (%)
Table 4.1 Biosimilar mAbs on the Market in 2017: Revenues ($m) and Market Shares (%)
Table 4.2 Remsima/Inflectra/Flammegis (Infliximab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2016-2028
Table 4.3 Infimab (Infliximab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2016-2028
Table 4.4 Reditux (Rituximab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2016-2027
Table 4.5 Mabtas (Rituximab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2016-2028
Table 4.6 Biocad Distribution Partners for AcellBia, 2017
Table 4.7 AcellBia (Rituximab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2016-2028
Table 4.8 MABALL (Rituximab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2016-2028
Table 4.9 Clotinab (Abciximab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2016-2028
Table 4.10 AbcixiRel (Abciximab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2016-2028
Table 4.11 Herzuma (Trastuzumab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2016-2028
Table 4.12 CANMAb/Hertraz (Trastuzumab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2016-2028
Table 4.13 BI695500 (Rituximab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2016-2028
Table 4.14 CT-P10 (Rituximab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2018-2027
Table 4.15 BI695501 (Adalimumab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2017-2028
Table 4.16 BCD-022 (Trastuzumab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2016-2028
Table 4.17 FKB327 (Adalimumab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2017-2028
Table 4.18 FKB238 (Bevacizumab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2020-2028
Table 4.19 BCD-021 (Bevacizumab) Forecast: Revenue ($m), Market Share (%), AGR (%) and CAGR (%), 2018-2028
Table 5.1 Leading National Markets for the Global Biosimilar mAb Industry, 2017
Table 5.2 Leading National Markets for the Global Biosimilar mAb Industry, 2017, 2023, 2028
Table 5.3 The Indian Biosimilar MAb Market: Revenue ($m), Annual Growth (%) and CAGR (%) Forecast, 2016-2028
Table 5.4 The Russian Biosimilar mAb Market: Revenue ($m), Annual Growth (%) and CAGR (%) Forecast, 2016-2028
Table 5.5 The South Korean Biosimilar mAb Market: Revenue ($m), Annual Growth (%) and CAGR (%) Forecast, 2016-2028
Table 5.6 The Chinese Biosimilar mAb Market: Revenue ($m), Annual Growth (%) and CAGR (%) Forecast, 2016-2028
Table 5.7 The Brazilian Biosimilar mAb Market: Revenue ($m), Annual Growth (%) and CAGR (%) Forecast, 2016-2028
Table 5.8 The EU5 Biosimilar mAb Market: Revenue Forecast ($m), 2016-2028
Table 5.9 The German Biosimilar mAb Market: Revenue ($m), Annual Growth (%) and CAGR (%) Forecast, 2016-2028
Table 5.10 The French Biosimilar mAb Market: Revenue ($m), Annual Growth (%) and CAGR (%) Forecast, 2016-2028
Table 5.11 The UK Biosimilar mAb Market: Revenue ($m), Annual Growth (%) and CAGR (%) Forecast, 2016-2028
Table 5.12 The Italian Biosimilar mAb Market: Revenue ($m), Annual Growth (%) and CAGR (%) Forecast, 2016-2028
Table 5.13 The Spanish Biosimilar mAb Market: Revenue ($m), Annual Growth (%) and CAGR (%) Forecast, 2016-2028
Table 5.14 The Japanese Biosimilar mAb Market: Revenue ($m), Annual Growth (%) and CAGR (%) Forecast, 2016-2028
Table 5.15 Finalised FDA Biosimilar Guidelines, 2015
Table 5.16 The US Biosimilar mAb Market: Revenue ($m) and Annual Growth (%) Forecast, 2017-2028
Table 5.17 The Mexico Biosimilar mAb Market: Revenue ($m) and Annual Growth (%) Forecast, 2017-2028
Table 5.17 The Canada Biosimilar mAb Market: Revenue ($m) and Annual Growth (%) Forecast, 2017-2028
Table 6.1 Companies in the Biosimilar mAb Industry by Number of Compounds in Development, 2017
Table 6.2 Selected Collaborations for Biosimilar mAb Development, 2009-2017
Table 6.3 BioXpress: Compounds in the Pipeline, 2018
Table 6.4 Tests Carried Out by BioXpress During Biosimilar mAb Development, with Objectives
Table 6.5 Compounds in Celltrion’s Pipeline: Compound Name, Generic Name, Development Stage and Anticipated Year of Approval, 2017
Table 6.6 Genor Biopharma: Biosimilar mAbs Pipeline, 2017
Table 6.7 Mabion: Compounds in the Pipeline and Their Development Stage, 2017
Table 6.8 Gene Techno Science: Revenue ($m), from 2013-2017
Table 6.9 Biocon: Compounds in the Pipeline and Their Stage of Development, 2017
Table 6.10 Big Pharma's Positions in the Biosimilar mAb Industry, 2017
Table 7.1 Strengths and Weaknesses of the Biosimilar mAb Market, 2016-2028
Table 7.2 Opportunities and Threats Facing the Biosimilar mAb Market, 2016-2028
Table 7.3 Social and Technological Factors Influencing the Biosimilar mAb Market, 2016-2028
List of Figures
Figure 1.1 Main Compound Classes of Biosimilar mAbs, 2018
Figure 2.1 Structure of an Antibody
Figure 3.1 Global Biosimilar mAbs Market Forecast: Revenue ($m), 2016-2028
Figure 3.2 The Biosimilar mAb Market: Market Share (%) by compound in 2017
Figure 3.3 The Biosimilar mAb Market: Market Share (%) by compound in 2023
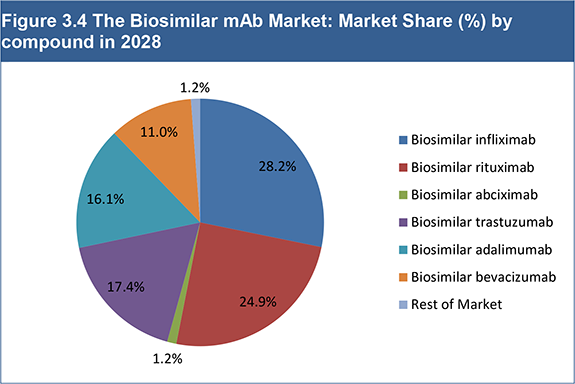
Figure 3.4 The Biosimilar mAb Market: Market Share (%) by compound in 2028
Figure 3.5 Global Biosimilar Infliximab Market Forecast: Revenue ($m), 2016-2028
Figure 3.6 Global Biosimilar Rituximab Market Forecast: Revenue ($m), 2016-2028
Figure 3.7 Global Biosimilar Abciximab Market Forecast: Revenue ($m), AGR (%), and CAGR (%)
Figure 3.8 Global Biosimilar Trastuzumab Market Forecast: Revenue ($m), 2016-2028
Figure 3.9 Global Biosimilar Adalimumab Market Forecast: Revenue ($m), 2016-2028
Figure 3.10 Global Biosimilar Bevacizumab Market Forecast: Revenue ($m), 2016-2028
Figure 3.11 Drivers and Restraints for the Global Biosimilar mAb market 2016-2028
Figure 4.1 Biosimilar mAb Versions on the Market: Market Share (%) 2017
Figure 4.2 Biosimilar mAb Versions on the Market: Market Share (%) 2023
Figure 4.3 Biosimilar mAb Versions on the Market: Market Share (%) 2028
Figure 4.4 Remsima/Inflectra/Flammegis (Infliximab): Revenue Forecast ($m), 2016-2028
Figure 4.5 Remsima/Inflectra/Flammegis (Infliximab): AGR Forecast (%), 2016-2028
Figure 4.6 Infimab (Infliximab): Revenue Forecast ($m) 2016-2028
Figure 4.7 Infimab (Infliximab): AGR Forecast (%) 2016-2028
Figure 4.8 Reditux (Rituximab): Revenue Forecast ($m) 2016-2028
Figure 4.9 Reditux (Rituximab): AGR Forecast (%) 2016-2028
Figure 4.10 Mabtas (Rituximab): Revenue Forecast ($m) 2016-2028
Figure 4.11 Mabtas (Rituximab): AGR Forecast (%) 2016-2028
Figure 4.12 AcellBia (Rituximab): Revenue Forecast ($m) 2016-2028
Figure 4.13 AcellBia (Rituximab): AGR Forecast(%) 2016-2028
Figure 4.14 MABALL (Rituximab): Revenue Forecast ($m) 2016-2028
Figure 4.15 MABALL (Rituximab): AGR Forecast (%) 2016-2028
Figure 4.16 Clotinab (Abciximab): Revenue Forecast ($m) 2016-2028
Figure 4.17 Clotinab (Abciximab): AGR Forecast (%) 2016-2028
Figure 4.18 AbcixiRel (Abciximab): Revenue Forecast ($m) 2016-2028
Figure 4.19 AbcixiRel (Abciximab): AGR Forecast (%) 2016-2028
Figure 4.20 Herzuma (Trastuzumab): Revenue Forecast ($m) 2016-2028
Figure 4.21 Herzuma (Trastuzumab): AGR Forecast (%) 2016-2028
Figure 4.22 CANMAb/Hertraz (Trastuzumab): Revenue Forecast ($m) 2016-2028
Figure 4.23 CANMAb/Hertraz (Trastuzumab): AGR Forecast (%) 2016-2028
Figure 4.24 BI695500 (Rituximab): Revenue Forecast ($m) 2018-2028
Figure 4.25 BI695500 (Rituximab): AGR Forecast (%) 2019-2028
Figure 4.26 CT-P10 (Rituximab): Revenue Forecast ($m) 2019-2028
Figure 4.27 CT-P10 (Rituximab): AGR Forecast (%) 2019-2028
Figure 4.28 BI695501 (Adalimumab): Revenue Forecast ($m) 2018-2028
Figure 4.29 BI695501 (Adalimumab): AGR Forecast (%) 2019-2028
Figure 4.30 BCD-022 (Trastuzumab): Revenue Forecast ($m) 2016-2028
Figure 4.31 BCD-022 (Trastuzumab): AGR Forecast (%) 2017-2028
Figure 4.32 FKB327 (Adalimumab): Revenue Forecast (%) 2018-2028
Figure 4.33 FKB327 (Adalimumab): AGR Forecast (%) 2019-2028
Figure 4.34 FKB238 (Adalimumab): Revenue Forecast ($m) 2020-2028
Figure 4.35 FKB238 (Adalimumab): AGR Forecast (%) 2022-2028
Figure 4.36 BCD-021 (Bevacizumab): Revenue Forecast ($m) 2017-2028
Figure 4.37 BCD-021 (Bevacizumab): AGR Forecast (%) 2018-2028
Figure 5.1 The Biosimilar MAb Market by Country: Market Shares (%) of Revenue, 2017
Figure 5.2 The Biosimilar MAb Market: National Submarket Shares (%), 2023
Figure 5.3 The Biosimilar MAb Market: National Submarket Shares (%), 2028
Figure 5.4 The Indian Biosimilar mAb Market: Revenue Forecast ($m), 2016-2028
Figure 5.5 The Russian Biosimilar mAb Market: Revenue Forecast ($m), 2016-2028
Figure 5.6 The South Korean Biosimilar mAb Market: Revenue Forecast ($m), 2016-2028
Figure 5.7 The Chinese Biosimilar mAb Market: Revenue Forecast ($m), 2016-2028
Figure 5.8 The Brazilian Biosimilar mAb Market: Revenue Forecast ($m), 2016-2028
Figure 5.9 The EU5 Biosimilar mAb Market: Grouped Revenue Forecast ($m), 2016-2028
Figure 5.10 The EU5 Biosimilar mAb Market: Revenue Forecasts ($m) by Country, 2016-2028
Figure 5.11 The German Biosimilar mAb Market: Revenue Forecast ($m), 2016-2028
Figure 5.12 The French Biosimilar mAb Market: Revenue Forecast ($m), 2016-2028
Figure 5.13 The UK Biosimilar mAb Market: Revenue Forecast ($m), 2016-2028
Figure 5.14 The Italian Biosimilar mAb Market: Revenue Forecast ($m), 2016-2028
Figure 5.15 The Spanish Biosimilar mAb Market: Revenue Forecast ($m), 2016-2028
Figure 5.16 The Japanese Biosimilar mAb Market: Revenue Forecast ($m), 2016-2028
Figure 5.17 Biosimilar mAbs in the US: Revenue Forecast ($m), 2017-2028
Figure 5.18 Biosimilar mAbs in the Mexico: Revenue Forecast ($m), 2016-2028
Figure 5.19 Biosimilar mAbs in the Canada: Revenue Forecast ($m), 2016-2028
Figure 6.1 Prominent Companies in the Biosimilar mAb Industry: Share (%) of the Pipeline (2017)
Figure 8.1 Prominent Compounds in the Biosimilar mAb Market: Comparison of Revenues ($m), 2017, 2023 and 2028
Figure 8.2 Forecasted Biosimilar mAbs: Comparison of Revenues ($m), 2017, 2023 and 2028
Figure 8.3 Leading National Markets: Revenues ($m), 2017, 2023 and 2028
Figure 8.4 Prominent Companies in the Biosimilar mAb Industry: Number of Products in Current Development or in the Pipeline, 2017
Abbott
AbbVie
Aché
Actavis
AET BioTech
Alexion Pharmaceuticals
Allergan
Alvogen
Alvotech
Amgen
Apotex
Aprogen
Array Bridge
AstraZeneca
Aurobindo Pharma Limited
Axis Biotec Brasil
Baliopharm GmbH
Baxalta
Bayer
Binex
BIO
Bio Farma
BIOCAD
Biocerus
Biocon
Biogen Idec
Bio-Manguinhos
Bionovis
BioXpress Therapeutics
Boehringer Ingelheim
Bristol-Myers Squibb
Catalent Pharma Solutions
Cell Therapeutics
Celltrion
Celon Pharma Łomianki
Chugai
CinnaGen
CinnaGen
Coherus BioSciences
Daiichi Sankyo
DM Bio
Dong-A Pharma
Dr Reddy's
Dyadic International, Inc.
Egis Pharmaceuticals
Eisai
Eli Lilly
Emcure
EMS
Epirus Biopharmaceuticals
Farmasa
Fresenius
Fujifilm
Fujifilm Kyowa Kirin Biologics
Gedeon Richter
Gene Techno Science
Genentech
GeneTechno Science
Genexo
Genor Biopharma
GlaxoSmithKline
Glycotope
Hanwha Chemical
Harvest Moon
Hetero Group
Hospira
Hypermarcas
iBio
IBSS Biomed
ImClone LLC
Instas Pharmaceuticals
Instituto Vital Brazil
Intas Biopharmaceuticals
IPCA Labs
Isu Abxis
Janssen
JHL Biotech
Johnson & Johnson
Kyowa Hakko Kirin
Laboratorio Elea
Laboratorios Liomont
LG Life Sciences
Lonza
Mabion
Mabtech Limited
mAbxience
MedImmune
Merck & Co.
Merck (MSD)
Merck Serono
Millhouse LLC
Mitsubishi Tanabe
Mochida Pharmaceutical
Momenta Pharmaceuticals
Mustafa Nevzat Pharmaceuticals
Mylan
Natco Pharma
Nichi-Iko Pharmaceutical Co.
Nippon Kayaku
Novartis
Oncobiologics
Otsuka Pharmaceutical
Parexel
Paul-Ehrlich Institute of Germany
Pfizer
PharmaPraxis
Pharmstandard
PhRMA
PlantForm
Polfarmex
ProBioGen
Probiomed
QuantiaMD
Quintiles
Ranbaxy Laboratories
Regeneron Pharmaceuticals
Reliance GeneMedix
Reliance Life Sciences
Roche
Samsung Bioepis
Samsung Biologics
Sandoz
Sanofi
Schnell
Seattle Genetics
Shanghai CP Guojian
Shionogi
Sorrento Therapeutics
Spectrum Pharmaceuticals
Stada Arzneimittell AG
Synthon
Takeda
Teva Pharmaceutical
TL Biopharmaceutical AG
UCB
União Química
Viropro
Walvax Biotechnology
Wyeth
Zenotech Laboratories
Zhejiang HISUN Pharmaceuticals Co. Ltd. (HISUN)
Zhejiang Huahai Pharmaceutical
Zydus-Cadila
List of Organizations Mentioned in the Report
Agence française de sécurité sanitaire des produits de santé (ANSM)
Agência Nacional de Vigilância Sanitária (ANVISA)
Central Drugs Standard Control Organisation and the Department of Biotechnology
Chinese Centre for Drug Evaluation (CDE)
Cour des Comptes (France)
European Medicines Agency (EMA)
Food and Drug Administration (US FDA)
Fraunhofer Center for Molecular Biology
Health Canada
India Brand Equity Foundation
Israel Institute for Biological Research (IIBR)
Korean Food and Drug Administration (KFDA)
Medicines and Healthcare Products Regulatory Agency (MHRA)
Ministry of Food and Drug Safety (South Korea)
Ministry of Health of the Russian Federation
Ministry of Health, Labour and Welfare (MHLW)
National Institute for Health and Care Excellence (NICE)
National Institute for Health Research Horizon Scanning Centre
Norwegian Medical Agency
Scientific Centre for Expertise of Medicinal Application Products (Russia)
Spanish Ministry of Health
State Food and Drug Administration (SFDA)
The Cancer Centre Bahamas
The European Medicines Agency (EMA)
The German Generics Association Pro Generika
UCAB (The Utrecht Centre of Excellence for Affordable Biotherapeutics for Public Health
Washington Legal Foundation
World Health Organization (WHO)
Download sample pages
Complete the form below to download your free sample pages for Global Biosimilar Monoclonal Antibodies Forecast 2018-2028
Related reports
-

Vaccine Sales Market Forecast 2018-2028
The Global Vaccines Sales market was valued at $36.9 billion in 2017. This value will grow to $61.1bn in 2022...
Full DetailsPublished: 09 March 2018 -

Top 50 Bioreactor Manufacturers 2019
Asia-Pacific bioreactors market is anticipated to be the fast-growing market in the forecast period with a CAGR of 8.0% from...
Full DetailsPublished: 25 March 2019 -

Top 25 Antibiotic Drugs Manufacturers 2018
Visiongain forecasts the antibiotic drugs market to increase to $ 43,841.7m in 2022. The market is estimated to grow at...
Full DetailsPublished: 12 June 2018 -

Global Stem Cell Technologies and Applications Market 2018-2028
The global stem cell technologies and applications market is estimated to have reached $12,040 million and is expected to grow...
Full DetailsPublished: 02 March 2018 -

Global Inflammatory Bowel Diseases (IBD) Drug Market Forecast 2018-2028
The global inflammatory bowel diseases (IBD) drug market is estimated at $6.7bn in 2017 and $7.6bn in 2023. Biologic therapies...
Full DetailsPublished: 29 May 2018 -

Global Vaccine Contract Manufacturing Market Report 2018-2028
The global vaccine contract manufacturing market was worth $883.0m in 2017 and is expected to grow at a CAGR of...
Full DetailsPublished: 06 March 2018 -

Global Cancer Immunotherapy Market Forecast 2019-2029
The world cancer immunotherapy market is expected to grow at a CAGR of 11.4% in the second half of the...Full DetailsPublished: 21 January 2019 -

mRNA Vaccines and Therapeutics Market Forecast 2019-2029
The mRNA Vaccines and Therapeutics Market is estimated at $3.43 billion in 2018. The Standardized Therapeutic Cancer mRNA Vaccines segment...
Full DetailsPublished: 07 May 2019 -

Global Acute Myeloid Leukaemia Market Forecast 2018-2028
The global acute myeloid leukaemia market reached $1bn in 2017 and is estimated to reach $2.8bn by 2022. In 2017,...
Full DetailsPublished: 21 September 2018 -

Cell-Based Assays World Industry and Market 2018-2028
The revenue of the cell-based assays market in 2017 is estimated at $13bn and is expected to grow at a...Full DetailsPublished: 18 September 2018
Download sample pages
Complete the form below to download your free sample pages for Global Biosimilar Monoclonal Antibodies Forecast 2018-2028
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Don’t Miss Out!
Subscribe to receive the latest Industry news, trending insight and analysis straight to your inbox.Choose your preferences:
Latest Pharma news
Retinal Gene Therapy Market
The global Retinal Gene Therapy market is projected to grow at a CAGR of 9.6% by 2034
26 July 2024
HIV Drugs and Injectables Market
The global HIV Drugs & Injectables market is projected to grow at a CAGR of 4.6 % by 2034
24 July 2024
Digital Twin Technology in Pharmaceutical Manufacturing Market
The global Digital Twin Technology in Pharmaceutical Manufacturing market is projected to grow at a CAGR of 31.3% by 2034
23 July 2024
Specialty Pharma Market
The global Specialty Pharma market is projected to grow at a CAGR of 7.5% by 2034
22 July 2024

















