Industries > Pharma > Global Biosimilars and Follow-On Biologics Market 2018-2028
Global Biosimilars and Follow-On Biologics Market 2018-2028
Monoclonal Antibodies (mAbs), Fusion Proteins, Insulin, Erythropoietins, Granulocyte Colony-Stimulating Factor (G-CSF), Interferons, Growth Hormones, Fertility Hormones
The global biosimilars and follow-on biologics market is estimated to have reached $7.70bn in 2017 and expected to grow at a CAGR of 23.8% in the first half of the forecast period. The market is dominated by Biosimilar Monoclonal Antibodies, this submarket is estimated to hold 24% share of this market by 2022.
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in this sector.
In this brand new 335-page report you will receive 131 tables and 68 figures– all unavailable elsewhere.
The 335-page report provides clear detailed insight into the global biosimilars and follow-on biologics market. Discover the key drivers and challenges affecting the market.
By ordering and reading our brand-new report today you stay better informed and ready to act.
Report Scope
• Global Biosimilars and Follow-on Biologics Market forecasts from 2018-2028
• Along with revenue prediction for the overall world market for biosimilars, our investigation shows forecasts to 2028 for 8 individual therapeutic submarkets:
• Monoclonal antibodies (mAbs)
• Fusion proteins
• Insulin
• Erythropoietin (EPO)
• Granulocyte colony-stimulating factor (G-CSF)
• Interferons
• Growth hormones
• Fertility hormones
• This report also shows revenue to 2028 for 12 individual submarkets within the above segments:
• Rituximab, infliximab, trastuzumab, adalimumab and bevacizumab
• Human insulin, insulin analogues, insulin glargine and insulin lispro
• Interferon alfa and interferon beta
• Etanercept
• Our analyses show individual revenue forecasts to 2028 for 12 national markets:
• US
• Japan
• Germany, France, UK, Italy and Spain
• China, India, South Korea, Russia and Brazil
• Our study provides a SWOT analysis of the biosimilars and follow-on biologics market.
• Our study discusses pressures, opportunities and other events affecting the biosimilars industry and market, including these influences:
• Strategies for developing biosimilars – needs, demand, challenges and opportunities
• Guidelines from regulators (FDA, EMA and others)
• Patent challenges and data exclusivity for biopharmaceuticals
• Needs and opportunities in developing biosimilar mAbs, including rising incidence of cancers and increasing demand for lower-cost biologicals
• Developments in technology and operations for biosimilar drug production.
Visiongain’s study is intended for anyone requiring commercial analyses for the biosimilars and follow-on biologics market. You find data, trends and predictions.
Buy our report today Global Biosimilars and Follow-On Biologics Market 2018-2028: Monoclonal Antibodies (mAbs), Fusion Proteins, Insulin, Erythropoietins, Granulocyte Colony-Stimulating Factor (G-CSF), Interferons, Growth Hormones, Fertility Hormones.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
1. Report Overview
1.1 Biosimilars Overview
1.2 Biosimilars Market Segmentation
1.3 Why You Should Read this Report
1.4 How this Report Delivers
1.5 Main Questions Answered by this Report
1.6 Who is this Report for?
1.7 Research and Analysis Methods
1.8 Frequently Asked Questions (FAQ)
1.9 Some Associated Reports
1.10 About Visiongain
2. An Introduction to Biosimilars and Biosimilar Drug Development
2.1 What are Biologics?
2.1.1 Biologics can be Very Effective but also Very Expensive
2.1.2 Brief History of Biological Drug Development
2.1.3 Why are Biologics the Most Lucrative Products in the Global Pharmaceutical Market?
2.2 What are Biosimilars?
2.3 Brief History of Biosimilars
2.4 What are Interchangeable Biological Products and how do They Differ from Biosimilars?
2.4.1 FDA Guidelines on Prescription of Interchangeables vs Biosimilars
2.4.2 Where does the EMA Stand with Regard to Interchangeables?
2.4.3 Where do the EU5 and other European Countries Stand with Regard to Automatic Substitution?
2.4.4 Why Would Nations Oppose Automatic Substitution of Reference Biologics with Biosimilars?
2.5 Main Segments of the Overall Biosimilars Market
3. The Global Biosimilars Market 2018-2028
3.1 The Global Biosimilars Market
3.2 Biosimilars as a Share of the Biologics Market, 2017
3.2.1 Seven of the Top Ten Best-Selling Drugs are Biologics
3.2.2 Outlook for the Overall Biologics Market
3.3 Global Biosimilars Market Forecast, 2016-2028
3.4 What will Drive Growth in the Biosimilars Market?
3.4.1 Biosimilars Can Bring about Savings of Billions - $44bn for the US by 2024 and $33bn for Europe by 2020
3.4.2 Over $67bn Worth of Biologic Patents due to Expire by 2020
3.4.3 US Approves First Biosimilar - Zarxio. Will this Open the Floodgates? Interchangeability Can Also Drive Growth
3.4.4 Other Developed and Emerging Markets Will also Provide Opportunities for Growth
3.4.5 Competitive Landscape Emerging for Biosimilars
3.5 What Factors Can Restrain Sales Growth in the Biosimilars Market?
3.5.1 Complexity of Biologics Means that Biosimilar Development Faces Many Challenges
3.5.2 Opposition from Originator Companies and Patent Issues
3.5.3 Fragmentation in the Market as Many Companies Chase the Same Targets
3.5.4 The Threat of Biobetters and Next-Generation Biologics
3.6 Summary of the Drivers and Restraints for the Global Biosimilars Market
3.7 Global Biosimilars Market Forecast by Submarket, 2016-2028
3.7.1 Leading Segments in the Biosimilars Market
3.7.2 How Will the Composition of the Market Change Over the Next Ten Years?
4. Outlook for Biosimilars in Leading Developed Markets, 2016-2028
4.1 The Leading National Biosimilar Submarkets
4.2 National Submarket Forecasts 2016-2028
4.3 How will the Regional Composition of the Global Market Change from 2016-2028?
4.4 US Biosimilars Market Outlook 2016-2028
4.4.1 Status of the US Market in 2014-2018
4.4.2 FDA Finalises Three New Guidelines on 351(k) Applications in April 2015
4.4.2.1 The History of the US’ Biosimilar Guidelines
4.4.3 FDA Finally Releases Guidance on Biosimilar Naming
4.4.4 Individual States Can Pass their Own Biosimilar Substitution Laws
4.4.5 US Biosimilars Market Forecast 2016-2028
4.5 The EU Biosimilar Market: History and Current Status
4.5.1 History of EMA Guidelines and Updates
4.5.2 Biosimilar Uptake in the Europe Varies between Nations
4.5.3 European Biosimilars Market Forecast, 2016-2028
4.5.4 Inflectra and Remsima in Europe - Demonstrates a Path for Other Biosimilar mAbs
4.5.5 German Biosimilar Market Outlook, 2018
4.5.5.1 German Biosimilar Market Forecast, 2016-2028
4.5.6 French Biosimilars Market Outlook, 2018
4.5.6.1 Biosimilar Substitution Passed but Requires Decrees to Come into Effect, Expected Sometime This Year
4.5.6.2 French Biosimilar Market Forecast, 2016-2028
4.5.7 UK Biosimilar Market Outlook, 2018
4.5.7.1 UK Biosimilar Market Forecast, 2016-2028
4.5.8 Italian Biosimilars Market Outlook, 2018
4.5.8.1 Italian Biosimilars Market Forecast, 2016-2028
4.5.9 Spanish Biosimilars Market Outlook, 2018
4.5.9.1 Spanish Biosimilar Market Forecast, 2016-2028
4.5.10 Biosimilar Regulation in Japan and Currently Approved Biosimilars
4.5.10.1 Differences between the European and Japanese Guidelines
4.5.10.2 Currently Approved Biosimilars in Japan
4.5.10.3 Japanese Biosimilar Market Forecast, 2016-2028
5. Outlook for Biosimilars in Emerging Markets, 2016-2028
5.1 China and India Lead Biosimilar Revenues
5.2 Leading Emerging Biosimilar Markets Forecast, 2016-2028
5.3 Chinese Biosimilars Market Outlook, 2018
5.3.1 China Publishes Final Guidelines for Biosimilars
5.3.2 Biosimilars Account for less than Half of Biotech Revenues in China
5.3.3 Chinese Biosimilars Market Forecast, 2016-2028
5.4 Indian Biosimilars Market Outlook, 2018
5.4.1 CDSCO Guidelines Released in 2012
5.4.2 Indian Biosimilars Market Forecast, 2016-2028
5.5 South Korea’s Established Biosimilar Guidelines
5.5.1 Currently Approved Biosimilars in South Korea, 2018
5.5.2 South Korean Biosimilars Market Forecast, 2016-2028
5.6 Russian Biosimilars Market Outlook and Forecast, 2016-2028
5.7 Brazilian Biosimilar Regulation
5.7.1 Government Eager to Promote Biosimilar Development, and Two Major Conglomerates, Bionovis and Orygen, are Racing to Produce Biosimilars
5.7.2 Brazilian Biosimilars Market Forecast, 2016-2028
6. Biosimilar Monoclonal Antibodies: Submarket Forecast and Pipeline, 2016-2028
6.1 MAbs as Part of the Overall Biologics Market in 2018
6.2 Monoclonal Antibodies (mAbs): Largest Biologic Submarket, with Individual Drugs Accumulating Multi-Billion Dollar Revenues
6.3 Biosimilar Monoclonal Antibodies Market and Submarkets Forecast, 2016-2028
6.4 Drivers for the Biosimilar Monoclonal Antibodies Market
6.4.1 New Launches of Biosimilar mAbs in Developed and Emerging Markets
6.4.2 Rising Incidence of Cancer Will Drive Demand
6.4.3 The Need for Lower Cost Therapies
6.4.4 Partnering to Launch Biosimilar mAbs
6.5 Restraints for the Monoclonal Antibodies Market
6.5.1 Novel mAb Developers Choosing to Develop Biobetters and Next-Generation Therapies in Face of Biosimilar Competition
6.5.1.1 Hope for Biosimilars when Competing Against Next-Generation Therapies
6.5.2 Challenges in Antibody Development and Manufacturing
6.5.3 Patent Issues, Market Fragmentation and Perception of Biosimilars
6.6 Leading Targets for Biosimilar Development, 2016-2028
6.7 Biosimilar Rituximab
6.7.1 Rituxan: The First Anti-Cancer mAb
6.7.2 Approved Biosimilars and Rituxan Patent Expiry
6.7.2.1 Reditux (Dr. Reddy’s Laboratories): First Biosimilar Rituximab
6.7.2.2 MabTas (Intas Pharmaceutical): Another Indian Biosimilar
6.7.2.3 AcellBia (Biocad): The Leading Biosimilar Rituximab
6.7.2.4 Kikuzubam (PROBIOMED) – Faced Challenges
6.7.3 Biosimilar Rituximab Pipeline, 2016-2028
6.7.3.1 Terminated Developments
6.7.3.2 GP2013 (Sandoz): One of the Biggest Names in the Biosimilar Industry Throws its Hat into the Ring with Advanced Stage Candidate
6.7.3.3 BI 695500 (Boehringer Ingelheim)
6.7.3.4 MabionCD20 (Mabion): Focusing on Markets with High Demand
6.7.3.5 CT-P10 (Celltrion)
6.7.3.6 PF-05280586 (Pfizer)
6.7.4 Biosimilar Rituximab: Revenue Forecast 2016-2028
6.8 Biosimilar Infliximab
6.8.1 Remicade: Second Only to Humira
6.8.2 Approved Biosimilars and Remicade Patent Expiry: Uncertainty in the US after FDA Rejection
6.8.2.1 Inflectra and Remsima (Hospira and Celltrion)
6.8.2.2 Celltrion Speeds Ahead with US FDA Filing, and Inflectra Approved in Australia and Canada
6.8.2.3 Inflimab and BOW015 (Epirus Biopharmaceuticals and Ranbaxy Laboratories)
6.8.3 Biosimilar Infliximab Pipeline, 2016-2028
6.8.3.1 SB2 (Samsung Bioepis): Has been Filed for EU Approval and Shows Positive Phase 3 Results
6.8.3.2 NI-071 (Nichi-Iko): Advanced Stages
6.8.3.3 ABP 710 (Amgen) and BX2922 (BioXpress Therapeutics)
6.8.4 Biosimilar Infliximab: Revenue Forecast 2016-2028
6.9 Biosimilar Trastuzumab
6.9.1 Herceptin: Another Jewel in Roche’s Cancer mAb Portfolio
6.9.2 Approved Biosimilars and Herceptin Patent Expiry
6.9.2.1 Hertraz and CanMab (Mylan and Biocon): The First to Win Approval
6.9.2.2 Herzuma (Celltrion)
6.9.3 Biosimilar Trastuzumab Pipeline, 2016-2028
6.9.3.1 BCD-022 (Biocad): Leading Biosimilar Rituximab Developer Tries its Hand at Trastuzumab
6.9.3.2 ABP 980 (Amgen and Allergan): CHMP Approval
6.9.3.3 PF-05280014 (Pfizer): FDA halts approval
6.9.3.4 SB3 (Samsung Bioepis) and BX2318 (BioXpress Therapeutics)
6.9.4 Biosimilar Trastuzumab: Revenue Forecast 2016-2028
6.10 Biosimilar Adalimumab
6.10.1 Humira – The World’s Best-Selling Prescription Drug
6.10.2 Approved Biosimilars and Humira Patent Expiry
6.10.2.1 Exemptia (Zydus Cadila): The First Biosimilar Adalimumab
6.10.3 Biosimilar Adalimumab Pipeline, 2016-2028
6.10.3.1 ABP 501 (Amgen and Allergan): Positive Phase 3 Results Released
6.10.3.2 GP2017 (Sandoz): Phase 3
6.10.3.3 BI 695501 (Boehringer Ingelheim)
6.10.3.4 SB5 (Samsung Bioepis)
6.10.4 Biosimilar Adalimumab: Revenue Forecast 2016-2028
6.11 Biosimilar Bevacizumab
6.11.1 Avastin: High Revenues and Late Patent Expiry
6.11.2 Biosimilar Bevacizumab Pipeline, 2016-2028
6.11.2.1 BCD-021 (Biocad)
6.11.2.2 ABP 215 (Amgen and Allergan): Approved
6.11.2.3 BI 695502 (Boehringer Ingelheim): Phase 3
6.11.2.4 PF-06439535 (Pfizer): Phase 3
6.11.3 Biosimilar Bevacizumab: Revenue Forecast, 2016-2028
7. Biosimilar Fusion Proteins: Submarket Forecast and Pipeline, 2016-2028
7.1 Scientific Background
7.2 Differentiating Fusion Proteins
7.3 Fusion Proteins as Part of the Overall Biologics Market
7.4 Biosimilar Fusion Proteins Market Forecast, 2016-2028
7.5 Biosimilar Etanercept, 2016-2028
7.5.1 Enbrel is the Leading Fusion Protein
7.5.2 Enbrel Granted Extended Patent Protection in the US, Patent has Expired in EU
7.5.3 Multiple Biosimilars Available in Emerging Markets
7.5.3.1 Brenzys / SB4 (Merck and Samsung Bioepis): First Product Approval to Result from Biosimilar Collaboration
7.5.3.2 Yi Sai Pu (Shanghai CP Guojian Pharmaceutical) and Qiangke (Shanghai Celgen Biopharmaceutical)
7.5.3.3 Etacept (Cipla) and Intacept (Intas Pharmaceuticals)
7.5.3.4 Davictrel / HD-203 (Hanwha Chemical and Merck KGaA)
7.5.4 Biosimilar Etanercept Pipeline
7.5.5 GP2015 (Sandoz)
7.5.5.1 Sandoz Goes in Early: FDA Accepts Application for GP2015, Despite the Fact that Enbrel Patents are not due to Expire any time Soon
7.5.6 SB4 (Samsung Bioepis): EMA Accepts Application
7.5.6.1 Samsung Bioepis Releases Positive Phase 3 Data for SB4
7.5.7 CHS-0214 (Coherus Biosciences and Baxter)
7.5.8 TuNEX / ENIA11 (Mycenax Biotech / TSH Biopharm Corp): Phase 3 in Taiwan
7.5.9 LBEC0101 (LG Life Sciences): Phase 3
7.5.10 Biosimilar Etanercept: Revenue Forecast 2016-2028
7.6 Eylea and Biosimilar Aflibercept
7.7 Orencia and Biosimilar Abatacept
8. Biosimilar Insulin Submarket Outlook, 2016-2028
8.1 Insulin as Part of the Biological Drug Sector
8.2 30 Million People Expected to be Diagnosed with Diabetes by 2030
8.3 Approved Insulin Biosimilars
8.4 Abasaglar / Basaglar / Insulin Glargine BS - The First Insulin Biosimilars to be Approved in Developed Markets
8.5 EMA Releases Finalised Insulin Biosimilars Guideline
8.6 Basaglar Delayed in the US until End of 2016
8.7 Biosimilar Insulin Market and Submarkets Forecast, 2016-2028
8.8 Biosimilar Human Insulin Submarket, 2016-2028
8.8.1 A Submarket Mostly Restricted to Emerging Markets?
8.8.3 Biosimilar Human Insulin in India
8.8.3.1 Wockhardt and Biocon - Given up on their Desire to Target the US and Europe?
8.8.4 Biosimilar Human Insulin in Russia
8.8.5 Biosimilar Human Insulin Submarket Forecast, 2016-2028
8.9 Biosimilar Insulin Analogues Submarket, 2016-2028
8.9.1 Available Biologic Insulin Analogues – Sanofi’s Lantus Tops Revenues
8.9.2 Insulin Glargine in the Main Biosimilar Target
8.9.2.1 Limited Development for the Other Targets
8.9.3 Insulin Market Leaders are Developing Ultra-Rapid Acting Insulin Analogues
8.9.3.1 Ultra-Long Acting Insulin also in Development
8.9.4 Mylan Partners with Biocon for Insulin Analogues
8.9.5 Other Biosimilar Insulin Analogue Revenue Forecast, 2016-2028
8.10 Biosimilar Insulin Glargine
8.10.1 Biosimilars in India and China
8.10.2 Biosimilar Insulin Glargine Pipeline
8.10.2.1 MK-1293 (Merck / Samsung Bioepis)
8.10.2.2 Mylan’s Insulin Glargine (Mylan and Biocon)
8.10.3 Biosimilar Insulin Glargine: Revenue Forecast, 2016-2028
8.11 Biosimilar Insulin Lispro
8.11.1 Currently Available Biosimilars
8.11.2 Much Smaller Pipeline than for Insulin Glargine
8.11.3 Biosimilar Insulin Lispro: Revenue Forecast, 2016-2028
9. Biosimilar Erythropoietins Submarket Outlook, 2016-2028
9.1 Three Products Lead the Biologic EPO Therapy Market
9.2 Safety Concerns for Erythropoietin-Stimulating Agents
9.3 The Challenge from Oral Therapies is Coming
9.4 Treating Anaemia in Patients with CKD - the Leading Use of EPO Therapies
9.5 Biosimilar Epoetin Approvals in Europe
9.6 Epoetin Alfa Pipeline for the European Market
9.7 Trends in Uptake for Biosimilar Epoetins in Europe
9.8 Epoetin Alfa Pipeline for the US - Companies Preparing for Launch
9.8.1 Pfizer’s Retacrit Delayed after Response Letter from FDA
9.8.2 Binocrit / HX575 has Completed Trials
9.9 Biosimilar Epoetin in Japan
9.10 Biosimilar Epoetin in South Korea
9.11 Biosimilar Epoetin in India and China
9.12 Second-Generation EPO Biosimilars
9.13 No Mircera Biosimilars in Clinical Development
9.14 Biosimilar EPO Market Forecast, 2016-2028
10. Biosimilar G-CSF Submarket Outlook, 2016-2028
10.1 Amgen Leads the Branded Biologic G-CSF Market
10.2 Teva Launches Long-Acting Pegfilgrastim
10.3 Selected Filgrastim Biosimilars Approved Worldwide
10.3.1 The European G-CSF Biosimilars Market
10.3.1.1 Trends in Biosimilar Uptake Across Europe
10.3.2 Tevagrastim (Teva): The First Biosimilar in Europe, Launched as Granix in the US
10.3.3 Nivestim (Hospira – Pfizer)
10.3.4 Zarzio (Sandoz): The Most Prescribed Biosimilar Therapy in Europe
10.3.5 Zarxio Becomes the First Biosimilar to be Approved in the US, Launched in September
10.3.6 Apotex: FDA has Accepted Applications for both its Biosimilar Filgrastim, and its Biosimilar Pegfilgrastim
10.3.7 Multiple Launches in Japan in Recent Years
10.3.8 Biosimilar Filgrastim and Pegfilgrastim in India
10.3.9 Biosimilars in Russia, China and Other Emerging Markets
10.4 Biosimilar G-CSF Market Forecast, 2016-2028
11. Biosimilar Interferons Submarket Outlook, 2016-2028
11.1 Interferons: Key Antiviral and MS Therapies Since the 1990s
11.2 Leading Interferon Brands
11.3 Competition for the Multiple Sclerosis Market - Challenges for Interferon Beta Therapies
11.4 All-Oral Regimens will Challenge Interferon Alfa Therapy
11.4.1 However Oral Therapies will be Expensive
11.5 There are no Approved Biosimilars in Developed Markets
11.6 Biosimilar Interferon Market Forecast, 2016-2028
11.7 Biosimilar Interferon Alfa
11.7.1 Biosimilar Interferon Alfa – a Common Target in Developing Countries, Leading to Fragmented Markets
11.7.2 Biosimilar Peginterferon Alfa
11.7.3 Hepatitis Treatment Rates are Low
11.7.4 Biosimilar Interferon Alfa Submarket Forecast, 2016-2028
11.8 Biosimilar Interferon Beta
11.8.1 Biosimilars are Well-Established in Emerging Markets
11.8.2 Biosimilar Interferon Beta Pipeline
11.8.3 EMA Guidance
11.8.4 Long-Acting Interferon Beta
11.8.5 Biosimilar Interferon Beta Submarket Forecast, 2016-2028
12. Biosimilar Recombinant Hormones Submarket Outlook, 2016-2028
12.1 Human Growth Hormone: First Extracted in 1958
12.2 Biologic Growth Hormone Market in 2014 - Novo Nordisk and Pfizer Dominate
12.2.1 Novo Nordisk Aiming to Retain Dominance Through FlexPro Device and NN8640 Somatropin Candidate
12.3 Biosimilar Growth Hormones
12.3.1 Omnitrope - Well Established in Developed Markets
12.4 Multiple Biosimilars Available Worldwide
12.4.1 Biosimilars Growth Hormones Outside of Asia
12.4.2 Biosimilar Growth Hormones in Asia
12.4.3 Biosimilar Uptake Varies by Region
12.5 Biosimilar Growth Hormones Market Forecast, 2016-2028
12.6 Fertility Hormones
12.6.1 Two Products Lead the Branded Fertility Hormone Market
12.6.2 Long-Acting Follicle Stimulating Hormone (FSH)
12.6.3 Branded Fertility Hormones Market Outlook
12.6.4 Available Biosimilar Fertility Hormones
12.6.5 Biosimilars Approved in Europe
12.6.5.1 Ovaleap (Teva)
12.6.5.2 Befomla (Finox Biotech) Approved in Europe, Undergoing Phase 3 for US
12.6.6 Rising Infertility to Drive Demand to 2028
12.6.7 Biosimilar Fertility Hormones Market Forecast, 2016-2028
13. Biosimilars: Qualitative Analysis and Industry Trends
13.1 SWOT Analysis of the Biosimilars Market
13.2 Biosimilars Market: Strengths
13.2.1 Biosimilars Can Offer Huge Cost Savings
13.2.2 The US has Finally Joined the Global Biosimilars Market
13.2.3 Biosimilars are Already Well-Established in Many National Markets
13.2.4 Outsourcing Offers the Chance for Further Cost Savings
13.3 Biosimilars Market: Weaknesses
13.3.1 Biosimilars are High Cost to Develop and Manufacture
13.3.2 Complexity Means that Development Timelines are Long
13.3.3 Uptake for Biosimilars Varies and is Low in Some Regions
13.4 Biosimilars Market: Opportunities
13.4.1 Blockbuster Biologics Face Patent Expiry
13.4.2 Rising Disease Prevalence
13.4.3 Licensing Agreements for Biosimilars
13.5 Threats
13.5.1 Biobetters and Next-Generation Biologics
13.5.2 Patent Issues and Product Life Cycle Management from Originator Companies
13.5.3 Fragmentation in the Market
13.5.4 Long Market and Data Exclusivity Periods
13.5.5 Biosimilars Require Marketing and Education
13.6 Biosimilars Product Pipeline
14. Conclusions from Visiongain’s Research and Analysis
14.1 Rapid Growth Expected for this High-Potential Market
14.2 Biosimilar Monoclonal Antibodies to be the Fastest Growing Segment
14.3 A Range of Factors Will Stimulate Demand
14.4 Challenges Remain in Developing and Successfully Launching Biosimilars
Associated Reports
Visiongain Report Sales Order Form
About Visiongain
Visiongain report evaluation form
List of Table
Table 2.1 First Approvals for Recombinant Protein Therapies, 1982-1993
Table 3.1 Top Ten Best-Selling Drugs of 2017 (in the Overall Pharmaceutical Market): Revenues ($bn), Market Shares (%)
Table 3.2 Global Biosimilars Market Forecast: Revenue ($bn), AGR (%),CAGR (%), 2016-2022
Table 3.3 Global Biosimilars Market Forecast: Revenue ($bn), AGR (%),CAGR (%), 2023-2028
Table 3.4 Patent Status for Leading Biologics within the Overall Biologics Market, 2018
Table 3.5 Biosimilars Market Forecast by Submarket (Insulin, EPO, G-CSF, Growth Hormones, mAbs): Revenue ($bn), AGR (%), CAGR (%), Market Shares (%), 2016-2022
Table 3.6 Biosimilars Market Forecast by Submarket (Interferons, Fusion Proteins, Fertility Hormones, Other Biosims): Revenue ($bn), AGR (%),CAGR (%), Market Shares (%), 2016-2022
Table 3.7 Biosimilars Market Forecast by Submarket (Insulin, EPO, G-CSF, Growth Hormones, mAbs): Revenue ($bn), AGR (%),CAGR (%), Market Shares(%), 2023-2028
Table 3.8 Biosimilars Market Forecast by Submarket (Interferons, Fusion Proteins, Fertility Hormones, Other Biosims): Revenue ($bn), AGR (%),CAGR (%), Market Shares (%), 2023-2028
Table 4.1 Global Biosimilars Market by Region: Revenues ($bn),
Market Share (%), 2018
Table 4.2 Global Biosimilars Market Forecast Segmented by Nation (China, EU, India, US, South Korea, Japan): Revenue ($bn), AGR (%),CAGR (%),Market Share(%), 2016-2022
Table 4.3 Global Biosimilars Market Forecast Segmented by Nation (Russia, Brazil, Other): Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 4.4 Global Biosimilars Market Forecast Segmented by Nation (China, EU, India, US, South Korea, Japan): Revenue ($bn), AGR (%),CAGR (%),Market Share(%) 2023-2028
Table 4.5 Global Biosimilars Market Forecast Segmented by Nation (Russia, Brazil,Other): Revenue ($bn), AGR (%), CAGR (%), Market Share(%), 2023-2028
Table 4.6 U.S. Biosimilar Approvals To-Date, 2018 Table 4.7 US Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 4.8 US Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 4.9 European Biosimilar Approvals: Monoclonal Antibodies and Insulin
Table 4.10 European Biosimilar Approvals: G-CSF
Table 4.11 European Biosimilar Approvals: EPO
Table 4.12 European Biosimilar Approvals: Growth Hormones and Fertility
Hormones
Table 4.13 European Biosimilars Market Segmented by Leading Nations: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 4.14 European Biosimilars Market Segmented by Leading Nations: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 4.15 German Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 4.16 German Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 4.17 French Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 4.18 French Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 4.19 UK Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 4.20 UK Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 4.21 Italian Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 4.22 Italian Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 4.23 Spanish Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 4.24 Spanish Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 4.25 Japanese Biosimilar Approvals To-Date, 2018
Table 4.26 Japanese Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 4.27 Japanese Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 5.1 Leading Emerging Biosimilar Markets: Revenue ($bn), AGR (%),
CAGR (%), Market Share (%), 2016-2022
Table 5.2 Leading Emerging Biosimilar Markets: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 5.3 Chinese Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 5.4 Chinese Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 5.5 Indian Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 5.6 Indian Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 5.7 Currently Approved Biosimilars in South Korea, 2016
Table 5.8 South Korean Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 5.9 South Korean Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 5.10 Russian Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 5.11 Russian Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 5.12 Brazilian Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2016-2022
Table 5.13 Brazilian Biosimilars Market: Revenue ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 6.1 The Best-Selling mAbs of 2017: Revenue ($bn), 2017
Table 6.2 Biosimilar Monoclonal Antibodies Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 6.3 Biosimilar Monoclonal Antibodies Market Forecast:
Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 6.4 Biosimilar Monoclonal Antibodies Market and Submarkets Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 6.5 Biosimilar Monoclonal Antibodies Market and Submarkets Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 6.6 Patent Status of Five Leading mAbs, 2018
Table 6.7 Types of Next-Generation Antibody Therapy, 2018
Table 6.8 Past and Present GP2013 (Sandoz) Clinical Trials, 2018
Table 6.9 Ongoing BI 695500 (Boehringer Ingelheim) Clinical Trials, 2018
Table 6.10 Ongoing CT-P10 (Celltrion) Clinical Trials, 2018
Table 6.11 Past and Present PF-05280586 (Pfizer) Clinical Trials, 2018
Table 6.12 Biosimilar Rituximab Revenue Forecast: Revenue ($bn), AGR (%),CAGR (%), 2016-2022
Table 6.13 Biosimilar Rituximab Revenue Forecast: Revenue ($bn), AGR (%),CAGR (%), 2023-2028
Table 6.14 Biosimilar Infliximab Revenue Forecast: Revenue ($bn), AGR (%),CAGR (%), 2016-2022
Table 6.15 Biosimilar Infliximab Revenue Forecast: Revenue ($bn), AGR (%),CAGR (%), 2023-2028
Table 6.16 Ongoing PF-05280014 (Pfizer) Clinical Trials, 2018
Table 6.17 Biosimilar Trastuzumab Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 6.18 Biosimilar Trastuzumab Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 6.19 Recent ABP 501 (Amgen and Allergan) Clinical Trials, 2018
Table 6.20 Ongoing BI 695501 (Boehringer Ingelheim) Clinical Trials, 2018
Table 6.21 Biosimilar Adalimumab Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 6.22 Biosimilar Adalimumab Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 6.23 Recent BCD-021 (Biocad) Clinical Trials, 2018
Table 6.24 Biosimilar Bevacizumab Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 6.25 Biosimilar Bevacizumab Revenue Forecast: Revenue ($bn), AGR (%),CAGR (%), 2023-2028
Table 7.1 Biosimilar Fusion Proteins Market Forecast: Revenue ($bn), AGR (%),CAGR (%), 2016-2022
Table 7.2 Biosimilar Fusion Proteins Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 7.3 Amgen’s US Patents and Expiry Dates for Enbrel
Table 7.4 Ongoing CHS-0214 (Coherus Biosciences and Baxter) Clinical Trials, 2018
Table 7.5 Biosimilar Etanercept Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 7.6 Biosimilar Etanercept Market Forecast: Revenue ($bn), AGR (%),CAGR (%), 2023-2028
Table 8.1 Diabetes Prevalence in Leading National Markets,
2012
Table 8.2 Selected Insulin Biosimilars which have been Approved and Launched
Table 8.3 Biosimilar Insulin Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 8.4 Biosimilar Insulin Market Forecast: Revenue ($bn), AGR (%),CAGR (%), 2023-2028
Table 8.5 Biosimilar Insulin Submarket Forecast: Revenue ($bn), AGR (%),CAGR (%), 2016-2022
Table 8.6 Biosimilar Insulin Submarket Forecast: Revenue ($bn), AGR (%),CAGR (%), 2023-2028
Table 8.7 Biosimilar Human Insulin Submarket Forecast: Revenue ($bn), AGR (%),CAGR (%), 2016-2022
Table 8.8 Biosimilar Human Insulin Submarket Forecast: Revenue ($bn), AGR (%),CAGR (%), 2023-2028
Table 8.9 Approved Insulin Analogues Marketed Worldwide, 2018
Table 8.10 Other Biosimilar Insulin Analogues Submarket Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 8.11 Other Biosimilar Insulin Analogues Submarket Forecast:
Revenue ($bn), AGR (%),CAGR (%), 2023-2028
Table 8.12 MK-1293 (Merck / Samsung Bioepis) Recent Clinical Trials, 2018
Table 8.13 Insulin Glargine Biosimilar (Mylan and Biocon) Ongoing Clinical Trials, 2018
Table 8.14 Biosimilar Insulin Glargine Revenue Forecast: Revenue ($bn), AGR (%),CAGR (%), 2016-2022
Table 8.15 Biosimilar Insulin Glargine Revenue Forecast: Revenue ($bn), AGR (%),CAGR (%), 2023-2028
Table 8.16 Biosimilar Insulin Lispro Revenue Forecast: Revenue ($bn), AGR (%),CAGR (%), 2016-2022
Table 8.17 Biosimilar Insulin Lispro Revenue Forecast: Revenue ($bn), AGR (%),CAGR (%), 2023-2028
Table 9.1 Ongoing ASP1517 (Astellas Pharma) Clinical Trials, 2018
Table 9.2 Binocrit / HX575 (Novartis) Clinical Trials, 2018
Table 9.3 Selected EPO Biosimilars Approved in India
Table 9.4 Biosimilar EPO Market Forecast: Revenue ($bn), AGR (%), CAGR (%),2016-2022
Table 9.5 Biosimilar EPO Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 10.1 Selected Filgrastim Biosimilars Approved Worldwide
Table 10.2 Biosimilar G-CSF Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 10.3 Biosimilar G-CSF Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 11.1 Types of Interferon Used Therapeutically, 2018
Table 11.2 Approved Branded Interferon Therapies, 2018
Table 11.3 Biosimilar Interferons Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 11.4 Biosimilar Interferons Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 11.5 Selected Interferon Alfa Biosimilars Approved Worldwide, 2018
Table 11.6 Chronic Hepatitis B and C: Global Prevalence by Region, 2014
Table 11.7 Biosimilar Interferon Alfa Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 11.8 Biosimilar Interferon Alfa Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 11.9 Selected Interferon Beta Biosimilars Approved Worldwide
Table 11.10 Biosimilar Interferon Beta Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 11.11 Biosimilar Interferon Beta Market Forecast: Revenue ($bn), AGR (%),CAGR (%), 2023-2028
Table 12.1 Biosimilar Growth Hormone Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 12.2 Biosimilar Growth Hormone Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 12.3 Recent Bemfola / Afolia (Finox Biotech) Clinical Trials, 2018
Table 12.4 Biosimilar Fertility Hormone Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2022
Table 12.5 Biosimilar Fertility Hormone Market Forecast: Revenue ($bn),AGR (%), CAGR (%), 2023-2028
Table 13.1 Expected Biologic Patent Expiries, 2016-2029
Table 13.2 Typical Models of Collaboration for Biosimilar Development
Table 13.3 Biosimilars product pipeline
Table 14.1 Biosimilars and Follow-On Biologics Market: Revenue ($bn) and Market Share (%) by Sector, 2018, 2022 and 2028
Table 14.2 Biosimilars and Follow-On Biologics Market: Revenue ($bn) and Market Share (%) by Region, 2018, 2022 and 2028
List of Figure
Figure 1.1 Main Segments and Sub-Segments of the Biosimilars Market, 2018
Figure 3.1 Biosimilars Market as Share of the Overall Biologics Market: Revenue ($bn), Market Share (%), 2017
Figure 3.2 Global Biosimilars Market Forecast: Revenue ($bn), 2016-2028
Figure 3.3 Drivers and Restraints for the Biosimilars Market, 2018
Figure 3.4 Global Biosimilars Market Forecasts by Submarket: Revenue ($bn), 2016-2028
Figure 3.5 Composition of the Biosimilars Market in 2018: Market Shares (%)
Figure 3.6 Composition of the Biosimilars Market in 2022: Market Shares (%)
Figure 3.7 Composition of the Biosimilars Market in 2028: Market Shares (%)
Figure 4.1 Regional Composition of the Biosimilars Market in 2018: Market Shares (%)
Figure 4.2 Global Biosimilars Market Forecast Segmented by Region: Revenue ($bn), 2016-2028
Figure 4.3 The Market Shares (%) for the Major Regional Markets 2016-2028
Figure 4.4 Regional Composition of the Biosimilars Market in 2022: Market Shares (%)
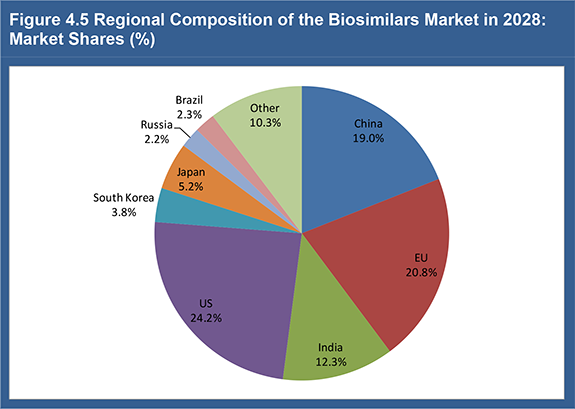
Figure 4.5 Regional Composition of the Biosimilars Market in 2028: Market Shares (%)
Figure 4.6 US Biosimilars Market Forecast: Revenue ($bn), 2016-2028
Figure 4.7 European Biosimilars Market Breakdown: Revenue ($bn), 2018
Figure 4.8 European Biosimilars Market Breakdown: Revenue ($bn), 2022
Figure 4.9 European Biosimilars Market Breakdown: Revenue ($bn), 2028
Figure 4.10 European Biosimilars Market Segmented by Leading Nations: Revenue ($bn), 2016-2028
Figure 4.11 German Biosimilars Market: Revenue ($bn), 2016-2028
Figure 4.12 French Biosimilars Market: Revenue ($bn), 2016-2028
Figure 4.13 UK Biosimilars Market: Revenue ($bn), 2016-2028
Figure 4.14 Italian Biosimilars Market: Revenue ($bn), 2016-2028
Figure 4.15 Spanish Biosimilars Market: Revenue ($bn), 2016-2028
Figure 4.16 Japanese Biosimilars Market: Revenue ($bn), 2016-2028
Figure 5.1 Leading Emerging Nations Biosimilar Market Shares: Revenues ($bn), 2018
Figure 5.2 Leading Emerging Nations Biosimilar Market Shares: Revenues ($bn), 2022
Figure 5.3 Leading Emerging Nations Biosimilar Market Shares: Revenues ($bn), 2028
Figure 5.4 Leading Emerging Nations Biosimilar Market Shares: Revenues ($bn), 2016-2028
Figure 5.5 Chinese Biosimilars Market: Revenue ($bn), 2016-2028
Figure 5.6 Indian Biosimilars Market: Revenue ($bn), 2016-2028
Figure 5.7 South Korean Biosimilars Market: Revenue ($bn),2016-2028
Figure 5.8 Russian Biosimilars Market: Revenue ($bn), 2016-2028
Figure 5.9 Brazilian Biosimilars Market: Revenue ($bn), 2016-2028
Figure 6.1 Monoclonal Antibodies as a Percentage of the Total Biologics Market: Revenue ($bn), Market Share (%), 2016
Figure 6.2 The Best-Selling mAbs of 2017 as a Percentage of the Total Biologics Market: Revenue ($bn), Market Share (%), 2017
Figure 6.3 Biosimilar Monoclonal Antibodies Market Forecast: Revenue ($bn), 2016-2028
Figure 6.4 Biosimilar Monoclonal Antibody Market Forecast, Split by Product: Revenue ($bn), 2016-2028
Figure 6.5 Biosimilar Rituximab Revenue Forecast: Revenue ($bn), 2016-2028
Figure 6.6 Biosimilar Infliximab Revenue Forecast: Revenue ($bn), 2016-2028
Figure 6.7 Biosimilar Trastuzumab Revenue Forecast: Revenue ($bn), 2016-2028
Figure 6.8 Biosimilar Adalimumab Revenue Forecast: Revenue ($bn),2016-2028
Figure 6.9 Biosimilar Bevacizumab Revenue Forecast: Revenue ($bn), 2016-2028
Figure 7.1 Fusion Proteins as a Percentage of the Total Biologics Market: Revenue ($m), Market Share (%), 2016
Figure 7.2 Biosimilar Fusion Proteins Market Forecast: Revenue ($bn), 2016-2028
Figure 7.3 Biosimilar Etanercept Market Forecast: Revenue ($bn), 2016-2028
Figure 8.1 Diabetes Prevalence in Leading National Markets, 2012
Figure 8.2 Biosimilar Insulin Market Forecast: Revenue ($bn), 2016-2028
Figure 8.3 Biosimilar Insulin Submarket Forecasts: Revenue ($bn), 2016-2028
Figure 8.4 Drivers and Restraints for the Biosimilar Insulin Market, 2018
Figure 8.5 Biosimilar Human Insulin Submarket Revenue Forecast: Revenue ($bn), 2016-2028
Figure 8.6 Other Biosimilar Insulin Analogues Submarket Forecast: Revenue ($bn), 2016-2028
Figure 8.7 Biosimilar Insulin Glargine Revenue Forecast: Revenue ($bn),2016-2028
Figure 8.8 Biosimilar Insulin Lispro Revenue Forecast: Revenue ($bn), 2016-2028
Figure 9.1 Biosimilar EPO Market Forecast: Revenue ($bn), 2016-2028
Figure 10.1 Biosimilar G-CSF Market Forecast: Revenue ($bn), 2016-2028
Figure 11.1 Biosimilar Interferons Market Forecast: Revenue ($bn), 2016-2028
Figure 11.2 Chronic Hepatitis B: Global Prevalence (%) by Region, 2014
Figure 11.3 Chronic Hepatitis C: Global Prevalence (%) by Region, 2014
Figure 11.4 Biosimilar Interferon Alfa Market Forecast: Revenue ($bn), 2016-2028
Figure 11.5 Biosimilar Interferon Beta Market Forecast: Revenue ($bn), 2016-2028
Figure 12.1 Biosimilar Growth Hormone Market Forecast: Revenue ($bn), 2016-2028
Figure 12.2 Biosimilar Fertility Hormone Market Forecast: Revenue ($bn), 2016-2028
Figure 13.1 Strengths and Weaknesses of the Biosimilars Market, 2016-2028
Figure 13.2 Opportunities and Threats for the Biosimilars Market, 2016-2028
Figure 13.3 Biosimilar Development Costs by Stage, 2018
Figure 13.4 Typical Biosimilar Development Timeline
Figure 14.1 Biosimilars and Follow-On Biologics Market: Revenue ($bn), by Sector, 2018, 2022 and 2028
Figure 14.2 Biosimilars and Follow-On Biologics Market: Revenue ($bn), by Region, 2018, 2022 and 2028
Allergan
Allozyne
Alteogen
Alvogen
Amarey Novamedical
Amega Biotech
Amgen
Apotex
Astellas Pharma
AstraZeneca
Avesthagen
Baxter
Bayer
Beijing Four Rings
Beijing SL Pharmaceutical
Biocad
Bioceuticals
Biogen
BioGenomics
Biolab
Bionovis
BioPartners
Biosidus
Bioton
BioXpress Therapeutics
Boehringer Ingelheim
Bristol-Myers Squibb
Cambridge Antibody Technology
CCL Pharmaceuticals
CCM Duopharma
Celltrion
Centocor Ortho Biotech
Chong Kun Dang
Chugai
CinnaGen.
Coherus BioSciences
Compass Biotechnologies
Cristália
CT Arzneimittel
Cyplasin
Daiichi Sankyo
Dong-A Pharmaceutical
Dr. Reddy’s Laboratories
Egis Pharmaceuticals
Eisai
Eli Lilly
Elpen Pharmaceutical
Emcure Pharmaceuticals
EMS
Epirus
Eurofarma
Express Scripts
FibroGen
Finox Biotech
Fuji Pharma
Gan & Lee
Genetech
Genexine
Gennova
GenSci
Genzyme
Geropharm
Gilead Sciences
GlaxoSmithKline
Hangzhou Jiuyuan Gene Engineering Co.
Hanmi Pharmaceutical
Hanwha Chemical
Haselmeier
Health Canada
Helius Biotech
Hexal
Hindustan Antibiotic
Hospira
Hualida Biotech
Hypermarcas
IGES Institute
Intas Biopharmaceuticals
inVentiv Health
JCR Pharmaceuticals
Johnson & Johnson
Kabi
Kemwell Biopharma
Kissea
Koçak Farma
Kwizda Pharma
Kyowa Hakko Kirin
Landsteiner Scientific
LG Life Sciences
Libbs
LKM SA
Lonza
Mabion
Marvel Life Sciences
MEDICE Arzneimittel Pütter
Merck (MSD)
Merck KGaA
Merck Serono
Minapharm
Mitsubishi Tanabe
Mochida Pharmaceutical
Momenta Pharmaceuticals
Mycenax Biotech
Mylan
Nichi-Iko
Nippion Kayaku
Novartis
Novo Nordisk
Nuron Biotech
Oncobiologics
Ortho Pharmaceutical
Orygen
PanGen Biotech
Pfenex
Pfizer
Pharmapark
Pharmstandard
PRA International
Pro Generika
PROBIOMED
Qilu Pharmaceutical
Quintiles
Ranbaxy Laboratories
RAND Corporation
Ratiopharm
Regeneron Pharmaceuticals
Reliance GeneMedix
Reliance Life Sciences (RLS)
Rentschler Biotechnologie
Roche
Samsung Bioepis
Samsung BioLogics
Sandoz
Sanofi
SciGen
Shandong Kexing Pharma
Shanghai Celgen Biopharmaceutical
Shanghai CP Guojian
Shanghai Fosun
Shantha Biotechnics
Shinogi
Sicor Biotech
Sothema Laboratories
Spectrum Pharmaceuticals
STADA Arzneimittel
Stragen Pharma
Strides Arcolab
Syngene International
Synthon Biopharmaceuticals
Takeda
Teva
Tianjin Hualida Biotechnology
Tonghua Dongbao
TSH Biopharm Corp
União Química
USV Biologics
Virchow Biotech
Wanbang Biopharmaceuticals
Wockhardt
Xiamen Amoytop Biotech
Zenotech
Zhejian Huahai Pharmaceutical
Zuventus
Zydus Biovation
Zydus Cadila
List of Organisations Mentioned in the Report
Argentine Ministry of Health
Cardiovascular and Renal Drugs Advisory Committee
Cardiovascular Drugs Advisory Committee
Centers of Medicare and Medicaid Services (CMS)
Central Drugs Standard Control Organisation
Centre for Drug Evaluation (CDE)
China Food and Drug Administration
Comisión Federal para la Protección contra Riesgos Sanitarios
Committee for Medicinal Products for Human Use
European Generic Medicines Association (EGA)
European Medicines Agency (EMA)
Intellectual Property Appellate Board (IPAB)
Korea Food and Drug Administration
Mexican Supreme Court
Ministry for Health Labour and Welfare (MHLW)
National Health Service (NHS)
National Institute for Health and Care Excellence (NICE)
Norwegian Ministry of Health
Pan American Health Organization
Pharmaceuticals and Medical Devices Agency (PMDA)
Russian Ministry of Health
Scottish Medicines Consortium (SMC)
Stanford University
State Employees’ Social Security and Social Services Institute (ISSSTE)
The Brazilian Ministry of Health
The Drug Regulatory Authority of Pakistan
The Ministry of Food and Drug Safety (MFDS)
The National Conference of State Legislatures (US)
The United Laboratories (TUL)
Therapeutic Goods Administration
Toronto University
UK Medicines and Healthcare Products Regulatory Agency (MHRA)
University of California, San Francisco
University of Tokyo
US Center for Disease Prevention and Control (CDC)
US Court of Appeals
US Food and Drug Administration (FDA)
US Patent and Trademark Office
World Health Organization (WHO)
Download sample pages
Complete the form below to download your free sample pages for Global Biosimilars and Follow-On Biologics Market 2018-2028
Related reports
-

Global Medical Device Contract Manufacturing Market Forecast 2018-2028
The global medical device contract manufacturing market was valued at $70bn in 2017. Visiongain forecasts this market to increase to...
Full DetailsPublished: 17 July 2018 -

Dermatological Drugs Market Forecast 2018-2028
The revenue of the dermatological drugs market in 2017 is estimated at $26.07bn and is expected to grow at a...
Full DetailsPublished: 14 August 2018 -

Global Biosimilar Monoclonal Antibodies Forecast 2018-2028
The global biosimilar monoclonal antibodies market is expected to reach $5.9bn in 2023 and is estimated to grow at a...
Full DetailsPublished: 27 February 2018 -

Pharma Leader Series: 25 Top Biosimilar Drug Manufacturers 2017-2027
Our 233-page report provides 126 tables, charts, and graphs, giving a clear view on companies in the biosimilar market. Who...Full DetailsPublished: 31 August 2017 -

Generic Drugs Market Forecast 2018-2028
The generic drugs market is estimated at $257.3bn in 2017 and is expected to grow at a CAGR of 7.9%...
Full DetailsPublished: 25 April 2018 -

Global Ophthalmic Devices Market 2018-2028
The global ophthalmic devices market is expected to grow at a CAGR of 5.5% in the first half of the...
Full DetailsPublished: 25 June 2018 -

Next-Generation Biologics Market 2018-2028
The introduction of new pipeline drugs in Next generation biologics, has led visiongain to publish this timey report. The Next...
Full DetailsPublished: 12 March 2018 -

Global Macular Degeneration (AMD) and Other Retinal Diseases Drugs Industry and Market 2018-2028
Our 171-page report provides 140 tables, charts, and graphs. Read on to discover the most lucrative areas in the industry...
Full DetailsPublished: 30 May 2018 -

Global OTC Pharmaceutical Market Forecast 2018-2028
In this brand new 201-page report you will receive 84 tables and 79 figures– all unavailable elsewhere. The 201-page report...
Full DetailsPublished: 05 April 2018 -

Pharmaceutical Contract Manufacturing Market 2018-2028
The pharmaceutical contract manufacturing market is expected to grow at a CAGR of 6.0% in the first half of the...
Full DetailsPublished: 27 June 2018
Download sample pages
Complete the form below to download your free sample pages for Global Biosimilars and Follow-On Biologics Market 2018-2028
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Don’t Miss Out!
Subscribe to receive the latest Industry news, trending insight and analysis straight to your inbox.Choose your preferences:
Latest Pharma news
Retinal Gene Therapy Market
The global Retinal Gene Therapy market is projected to grow at a CAGR of 9.6% by 2034
26 July 2024
HIV Drugs and Injectables Market
The global HIV Drugs & Injectables market is projected to grow at a CAGR of 4.6 % by 2034
24 July 2024
Digital Twin Technology in Pharmaceutical Manufacturing Market
The global Digital Twin Technology in Pharmaceutical Manufacturing market is projected to grow at a CAGR of 31.3% by 2034
23 July 2024
Specialty Pharma Market
The global Specialty Pharma market is projected to grow at a CAGR of 7.5% by 2034
22 July 2024