Industries > Pharma > Pharmaceutical Contract Manufacturing Market 2018-2028
Pharmaceutical Contract Manufacturing Market 2018-2028
Active Ingredient (API) and Finished Dose Formulation (FDF), Generic APIs, HPAPIs, Solid Dosages, Injectable Dosages
The pharmaceutical contract manufacturing market is expected to grow at a CAGR of 6.0% in the first half of the forecast period. The API Manufacturing submarket held 66% of the market in 2017. Visiongain estimated that the pharmaceutical contract manufacturing market will reach $93bn in 2022.
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in this sector.
In this brand new 230-page report you will receive 77 tables and 58 figures– all unavailable elsewhere.
The 230-page report provides clear detailed insight into the pharmaceutical contract manufacturing market. Discover the key drivers and challenges affecting the market.
By ordering and reading our brand-new report today you stay better informed and ready to act.
Report Scope
• Global Pharmaceutical Contract Manufacturing Market forecasts from 2018-2028
• Submarket forecasts at world level, from 2018-2028:
• Active pharmaceutical ingredients (APIs), with sub forecasts for generic APIs, high potency active pharma ingredients (HPAPIs) and other products
• Finished dosage formulations (FDFs), with sub forecasts for solid dose forms, injectable dosages and other dosage types
• Other applications of outsourced production – other related services
• Revenue forecasts from 2018-2028, for these regional and national markets:
• The US
• Canada
• Japan
• EU5: Germany, France, the UK, Italy and Spain
• BRIC: Brazil, Russia, India, China
• South Korea
• Turkey
• Mexico
• Others
• Assessment of selected leading companies that hold major market shares in the pharmaceutical contract manufacturing industry
• Qualitative Analysis from a CMO Perspective
• Qualitative Analysis from a Client Perspective
Visiongain’s study is intended for anyone requiring commercial analyses for the pharmaceutical contract manufacturing market. You find data, trends and predictions.
Buy our report today Pharmaceutical Contract Manufacturing Market 2018-2028: Active Ingredient (API) and Finished Dose Formulation (FDF), Generic APIs, HPAPIs, Solid Dosages, Injectable Dosages.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
1. Report Overview
1.1 Global Pharmaceutical Contract Manufacturing Market Segmentation
1.2 Global Pharmaceutical Contract Manufacturing Market Overview
1.3 Why You Should Read This Report
1.4 How This Report Delivers
1.5 Key Questions Answered by This Analytical Report
1.6 Who is This Report For?
1.7 Methodology
1.8 Frequently Asked Questions (FAQ)
1.9 Associated Visiongain Reports
1.10 About Visiongain
2. Introduction to Pharmaceutical Contract Manufacturing
2.1 What is Pharmaceutical Contract Manufacturing?
2.2 The Benefits of Outsourcing in the Pharmaceutical Market
2.3 Pharmaceutical Contract Manufacturing Services
2.4 Strategy and Tactics: The Importance of Outsourcing Wisely
2.5 Trends in Contract Manufacturing, 2017-2028
2.6 Pharmaceutical Contract Manufacturing: Market Segmentation
3. Pharmaceutical Contract Manufacturing: World Market 2017-2028
3.1 Pharmaceutical Contract Manufacturing Market: Historical Performance 2013-2017
3.1.1 Contract Manufacturing is the Most Mature Pharma Outsourcing Sector
3.1.2 The Pharma Contract Manufacturing Market by Sector 2017
3.2 Pharma Contract Manufacturing: Overall Market Forecast 2017-2028
3.2.1 Changing Market Shares by Segment 2017-2028
3.2.2 Drivers for Growth in the Contract Manufacturing Market to 2028
3.2.3 Generics: A Driver or Restraint?
3.2.4 Other Contract Manufacturing Services Market: Outlook and Forecast, 2017-2028
3.2.4.1 Increasing Demand for Formulation Development Services
4. The Contract API Manufacturing Services Submarket 2017-2028
4.1 The Contract API Manufacturing Submarket 2015-2017
4.1.1 Chemical APIs Will Experience Resurgence after the Shift of Focus to Biological APIs
4.1.2 The API Manufacturing Submarket by Sector, 2016
4.2 The Contract API Manufacturing Submarket: Outlook and Forecast 2017-2028
4.2.1 Drivers for Growth in the API Submarket to 2028
4.2.2 The Potential for Microreactors in Commercial Manufacturing to 2028
4.2.3 How Will New EU Regulations Impact the API Manufacturing Submarket?
4.2.4 Contract API Manufacturing Submarket Restraint 2017-2028
4.2.4.1 Backwards Integration for Generics Manufacturers
4.3 The Contract Generic API Manufacturing Submarket 2017-2028
4.3.1 The Contract Generic API Manufacturing Submarket Forecast 2017-2028
4.3.2 The Contract Generic API Manufacturing Submarket Drivers and Restraints to 2028
4.3.2.1 The US Generic Drug User Fee Act and API Manufacturing
4.4 The Contract HPAPI Manufacturing Submarket 2017-2028
4.4.1 Challenges and Opportunities in High Containment Manufacturing
4.4.2 Company Investment into HPAPI Manufacturing Facilities: Growth Increases in the HPAPI Manufacturing Sector
4.4.3 The Contract HPAPI Manufacturing Submarket Forecast 2017-2028
4.4.4 The Contract HPAPI Manufacturing Submarket Drivers and Restraints to 2028
5. The Contract FDF Manufacturing Services Submarket 2017-2028
5.1 The Contract FDF Manufacturing Submarket, 2017
5.1.1 Solid Dosage Manufacturing Leads the Market
5.1.2 CMOs Report High Demand for Softgel Formulations
5.2 The Contract FDF Manufacturing Submarket Forecast 2017-2028
5.2.1 Complex Molecule Manufacturing Will Drive Growth to 2028
5.2.2 What Will Restrain Growth in FDF Outsourcing?
5.3 The Contract Solid Dosage Manufacturing Submarket 2017-2028
5.3.1 How Will Revenue for Solid Dosage Manufacturing Grow to 2028?
5.3.2 The Solid Dosage Manufacturing Submarket Drivers and Restraints 2017-2028
5.3.2.1 Will Product Reformulation Drive Growth to 2028?
5.4 The Contract Injectable Dosage Manufacturing Submarket 2017-2028
5.4.1 The Contract Injectable Dosage Manufacturing Submarket Forecast 2017-2028
5.4.1.1 Drug Shortages Offer Opportunity to CMOs in the Short-Term
5.4.2 Demand for Biopharma Manufacturing Will Drive Growth 2017-2028
5.4.2.1 Interest in Antibody-Drug Conjugate Development Is Growing
5.4.2.2 Challenges in Manufacturing Antibody-Drug Conjugates
5.4.3 Lyophilisation and Aseptic Filling Demand Will Rise
5.4.3.1 Will Spray Drying Replace Lyophilisation in FDF Manufacturing?
6. Leading National Markets for Pharmaceutical Contract Manufacturing 2017-2028
6.1 Leading National Markets for Pharmaceutical Contract Manufacturing, 2017
6.1.1 Challenges in Assessing the Market: Supply versus Demand
6.1.2 Leading National Pharmaceutical Contract Manufacturing Markets: Revenue Forecasts 2017-2028
6.1.3 Pharmaceutical Manufacturing in the US: Regulatory Oversight, 2017
6.1.4 Controlling Foreign Manufacturing Sites
6.1.5 What Impact Will the GDUFA 2012 Have on Foreign Manufacturing?
6.1.6 Outsourcing and US Manufacturing Regulations
6.1.7 Increased Demand for Domestic API Manufacturing?
6.2 The US Contract Manufacturing Market Forecast 2017-2028
6.3 The Canada Contract Manufacturing Market Forecast 2017-2028
6.4 Outlook for Pharmaceutical Contract Manufacturing in the EU
6.4.1 The EU is the Leading Destination for Pharmaceutical Contract Manufacturing
6.4.2 Regulatory Aspects of Pharma Manufacturing in the EU
6.4.2.1 The European Commission Amends Annex 16
6.4.2.2 The Falsified Medicine Directive: Controlling API Imports
6.4.3 Biopharma Manufacturing Demand Will Rise to 2028
6.4.4 The EU Contract Manufacturing Market Forecast 2017-2028
6.4.5 Germany: The Leading EU Destination for API and FDF Manufacturing
6.4.5.1 Demand for Contract Manufacturing in Germany: Revenue Forecast 2017-2028
6.4.6 CMOs Account for a Third of Manufacturing Sites in France
6.4.6.1 French Pharma Contract Manufacturing Market Forecast 2017-2028
6.4.7 Italy: Strong API Manufacturing Traditions
6.4.7.1 Revenue Forecast for the Italian Pharma Contract Manufacturing Market 2017-2028
6.4.8 Spain: How Will Demand for Manufacturing Services Grow to 2028?
6.4.9 UK: Pharmaceutical Contract Manufacturing Market Forecast 2017-2028
6.5 Pharma Contract Manufacturing in Japan to 2028
6.5.1 Pharmaceutical Manufacturing Regulations in Japan
6.5.1.1 The Pharmaceutical Affairs Law
6.5.2 Greater API Outsourcing to Drive Japanese Market Growth to 2028 In Spite of Currency Devaluation Weakening the Economy
7. Leading Emerging Markets for Pharmaceutical Contract Manufacturing Market 2017-2028
7.1 Pharmaceutical Contract Manufacturing in Developing Countries
7.1.1 Outsourcing to Emerging National Markets from Developed Markets
7.1.1.1 Demand Will Increase for Services in India and China
7.1.2 Domestic Demand for Pharmaceutical Contract Manufacturing Services
7.1.3 Pharmaceutical Contract Manufacturing: Leading Emerging National Market Forecasts 2017-2028
7.2 Chinese Demand for Pharma Contract Manufacturing Services 2017-2028
7.2.1 Improved Manufacturing Regulations in China
7.2.1.1 Regulations for Excipients and Guidance for API Manufacturing
7.2.1.2 What Are the Consequences to Stricter Manufacturing Regulations in China?
7.2.2 Chinese CMOs Are Investing in Biopharma Manufacturing
7.2.3 China Pharma Contract Manufacturing Market Forecast 2017-2028
7.3 India: CMOs with Strong Infrastructure and Growing Expertise
7.3.1 Regulatory Outlook for Indian Pharma Manufacturing
7.3.1.1 Stricter Quality Standards to Ensure Compliance with EU Rules
7.3.2 Domestic Manufacturing Dominates the Indian Pharma Market
7.3.3 Demand for Contract Manufacturing in India: Market Forecast 2017-2028
7.4 Brazil: Contract Manufacturing Outlook 2017-2028
7.4.1 Pharmaceutical Manufacturing Regulations in Brazil
7.4.1.1 Guidelines and Regulations Proposed
7.4.2 Brazil Imports Most of its APIs
7.4.3 Brazil: Pharma Contract Manufacturing Market Forecast 2017-2028
7.5 The Russian Pharma Contract Manufacturing Market 2017-2028
7.5.1 Pharma Manufacturing Regulations in Russia
7.5.1.1 Compliance with GMP in Russia
7.5.2 How Will Demand for Contract Manufacturing Grow in Russia to 2028?
7.6 South Korea: Government Supported Growth
7.6.1 South Korea: Strict GMP Regulations
7.6.2 South Korea: Pharma Contract Manufacturing Market Forecast 2017-2028
7.7 Turkey: Domestic Market to offer Huge Growth Opportunities
7.7.1 Turkey: An Important Hub for Global Pharma Companies
7.7.2 Drug Approvals Delaying Growth of the Turkey Pharma Market
7.7.3 Turkey: Pharma Contract Manufacturing Market Forecast 2017-2028
7.8 Mexico: Emerging Market Power
7.8.1 Healthcare – A Top Priority in Mexico
7.8.2 Strategic Management of High Expectations
7.8.3 Mexico: Pharma Contract Manufacturing Market Forecast 2017-2028
8. Leading Companies in the Pharmaceutical Contract Manufacturing Market
8.1 Leading CMOs in the Pharmaceutical Contract Manufacturing Market
8.2 Catalent: Leading CMO
8.2.1 Multiple Expansions and Increased Focus on Biologic Manufacturing
8.2.2 Recent Developments
8.3 Lonza: Potential to Lead in ADC Manufacturing
8.3.1 Lonza: Expanding its Capacities to Meet Customer Demand for Biologic Manufacturing
8.3.2 Recent Developments
8.4 Evonik Degussa: Specialising in Small Molecules
8.4.1 Recent Developments
8.5 DPx Holdings - DSM and Patheon: Recent Merger Has Strengthened Their Position
8.5.1 Increasing Capacity Tactically and Expanding Worldwide
8.6 Teva: The World’s Largest API Manufacturer
8.6.1 Will Teva Expand into Emerging Markets?
8.6.2 Recent Developments
8.7 Boehringer Ingelheim BioXcellence: Boehringer’s Biopharma Manufacturing Division
8.7.1 Recent Developments
8.7.2 Adding New Services for Growth
8.8 Famar: A Possible Candidate for Acquisition
8.8.1 Recent Developments
8.9 Fareva: A Strategic Shift from Acquisition to Increase in Capacity
8.10 Vetter Pharma: Specialists in Aseptic Filling, Lyophilisation and Siliconisation
8.10.1 Vetter’s Growth: Increasing in Capacity and Market Expansion
8.11 Mylan: A World Leader in Generics Manufacturing
8.11.1 Recent Developments
9. Pharmaceutical Contract Manufacturing Industry Trends: Qualitative Analysis from a CMO Perspective 2017-2028
9.1 Pharma Contract Manufacturing Market: Strengths and Weaknesses from a CMO Perspective 2017-2028
9.2 Pharma Contract Manufacturing Market: Opportunities and Threats from a CMO Perspective 2017-2028
9.3 Pharma Contract Manufacturing Market: STEP Analysis 2017-2028
9.3.1 Social Factors
9.3.2 New Technologies Will Drive Market Growth to 2028
9.3.2.1 Advantages of Single-Use Technologies in Biopharma Manufacturing
9.3.2.2 Green Technology in Contract Manufacturing
9.3.2.3 Drug Delivery Trends and CMOs
9.3.2.4 Biologics Are Causing Increased Demand for Lyophilisation
9.3.2.5 Improving Solubility and Stability for Small Molecules
9.3.2.6 Spray Drying as an Advantageous Alternative to Lyophilisation
9.3.2.7 Hot-Melt Extrusion: A Niche Opportunity
9.3.3 Economic Pressures
9.3.3.1 Developed-Market CMOs Facing Competition from Emerging Markets
9.3.4 Political Issues
9.3.4.1 How Will Regulatory Developments Affect CMOs?
9.4 Trends in Drug Development Affecting CMOs to 2028
9.4.1 Orphan Drug Development: Smaller Manufacturing Capacities
9.4.2 Complex Biologics: Interest in ADCs is increasing
9.4.3 How Will Biosimilar Market Growth Affect CMOs?
9.4.4 The Future of Small Molecule Outsourcing
9.5 How Will CMOs Expand in the Coming 10 Years?
9.5.1 CMO Consolidation Will Increase
9.5.2 There Will Be More Manufacturing Facilities Available for Acquisition
9.6 Developments in the Manufacturing Process 2017-2028
9.6.1 Rising Interest from Companies in Quality by Design (QbD)
9.6.2 A Move Towards Continuous Manufacturing
10. Pharmaceutical Contract Manufacturing Trends: Qualitative Analysis of the Pharmaceutical Contract Manufacturing Market from a Client Perspective 2017-2028
10.1 Pharma Contract Manufacturing Market: Strengths and Weaknesses from a Client Perspective 2017-2028
10.2 Pharma Contract Manufacturing Market: Opportunities and Threats from a Client Perspective 2017-2028
10.3 Important Aspects of the Outsourcing Decision
10.3.1 What Factors Will Influence CMO Selection?
10.3.2 CMO - Pharma Partnering Models
10.3.3 Strategic Partnering
10.3.4 Selecting a Local CMO vs. Offshoring
10.3.5 CMOs in Emerging Markets
10.3.6 Good Communication is Vital for Success
10.3.7 Ensuring Protection in Intellectual Property and Tech Transfer
10.3.8 Regulatory Compliance is a Key Deciding Factor for Pharma Clients
10.3.8.1 Regulatory Bodies and Outsourcing
10.3.8.2 Quality Agreement
10.3.8.3 Global Harmonisation Will Improve Compliance
10.4 Trends in Outsourcing Partnerships 2017-2028
10.4.1 Big Pharma and Outsourced Manufacturing
10.4.2 Small and Virtual Pharma Companies
10.4.3 How Will Biotech Funding Impact the Pharma Contract Manufacturing Industry to 2028?
11. Conclusions
11.1 The World Pharmaceutical Contract Manufacturing Market
11.2 Outlook for the Market to 2028
11.2.1 Rising Demand for High Value Services 2017-2028
11.2.2 Demand Will Be Highest Among Developed-Market Clients
11.3 Which New Technologies Will Stimulate Demand to 2028?
Appendices
Associated Visiongain Reports
Visiongain Report Sales Order Form
About Visiongain
Visiongain report evaluation form
List of Tables
Table 3.1 Pharma Contract Manufacturing World Market Revenue ($bn), Annual Growth (%), CAGR (%), 2012-2016
Table 3.2 Pharma Contract Manufacturing Market: Revenue ($bn) and Market Share (%) by Sector, 2017
Table 3.3 Pharmaceutical Contract Manufacturing Market: Overall Market Forecasts by Sector, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 3.4 Pharma Contract Manufacturing Market: Market Shares (%), 2017-2022
Table 3.5Pharma Contract Manufacturing Market: Market Shares (%), 2023-2028
Table 3.6 Other Services Submarket Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 4.1 Contract API Manufacturing Submarket Revenue ($bn), Annual Growth (%), CAGR (%), 2012-2016
Table 4.2 Contract API Manufacturing Submarket: Revenue ($bn) and Submarket Share (%) by Sector, 2017
Table 4.3 Contract API Manufacturing Submarket: Overall Submarket Forecasts by Sector, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 4.4 Contract API Manufacturing Submarket Shares (%), 2017-2022
Table 4.5 Contract API Manufacturing Submarket Shares (%), 2022-2028
Table 4.6 Contract API Manufacturing Submarket: Drivers and Restraints, 2017-2028
Table 4.7 Contract Generic API Manufacturing Submarket Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 4.8 Contract Generic API Manufacturing Submarket: Drivers and Restraints, 2017-2028
Table 4.9 Contract HPAPI Manufacturing Submarket Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 4.10 Contract HPAPI Manufacturing Submarket: Drivers and Restraints, 2017-2028
Table 5.1 Contract FDF Manufacturing Submarket Revenue ($bn), Annual Growth (%), 2012-2016
Table 5.2 Contract FDF Manufacturing Submarket: Revenue ($bn) and Submarket Share (%) by Sector, 2017
Table 5.3 Contract FDF Manufacturing Submarket: Overall Submarket Forecasts by Sector, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 5.4 Contract FDF Manufacturing Submarket: Segment Shares (%), 2017-2022
Table 5.5 Contract FDF Manufacturing Submarket: Segment Shares (%), 2022-2028
Table 5.6 Contract FDF Manufacturing Submarket: Drivers and Restraints, 2017-2028
Table 5.7 Contract Solid Dosage Manufacturing Submarket Forecast, Revenue ($bn), Annual Growth (%), CAGR (%) 2017-2028
Table 5.8 Contract Solid Dosage Manufacturing Submarket: Drivers and Restraints, 2017-2028
Table 5.9 Contract Injectable Dosage Manufacturing Submarket Forecast, Revenue ($bn), Annual Growth (%), CAGR (%) 2017-2028
Table 5.10 Contract Injectable Dosage Manufacturing Submarket: Drivers and Restraints, 2017-2028
Table 5.11 Selected ADCs in Development, 2016
Table 5.12 Selected CMOs Investing in ADC Manufacturing Capacity
Table 6.1 Pharma Contract Manufacturing Market: Revenues ($bn) and Market Shares (%) by Region, 2017
Table 6.2 Pharma Contract Manufacturing Market: Regional Forecasts, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 6.3 Pharmaceutical Contract Manufacturing Market: Submarket Shares (%), 2017-2022
Table 6.4 Pharmaceutical Contract Manufacturing Market: Submarket Shares (%), 2022-2028
Table 6.5 GDUFA: Annual Fees for Domestic and Foreign Manufacturing Sites, 2015-2016
Table 6.6 US Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 6.7 Canada Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 6.8 EU Pharma Contract Manufacturing Market: Revenue ($bn) and Market Share (%) by Leading Country, 2017
Table 6.9 EU Pharma Contract Manufacturing Market: Revenue ($bn) and Market Share (%) by Leading Country, 2022
Table 6.10 EU Pharma Contract Manufacturing Market: Revenue ($bn) and Market Share (%) by Leading Country, 2028
Table 6.11 EU5 Pharma Contract Manufacturing Market: National Market Forecasts, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 6.12 German Pharma Contract Manufacturing: Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 6.13 Selected French Manufacturing Site Acquisitions, 2009-2013
Table 6.14 French Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 6.15 Italian Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 6.16 Spanish Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 6.17 UK Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 6.18 Japanese Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 7.1 Pharma Contract Manufacturing Market: Emerging Market Revenue ($bn) and Market Share (%) by Top Country, 2017
Table 7.2 Pharma Contract Manufacturing Market: Leading Emerging Market Forecasts ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 7.3 Pharma Contract Manufacturing Market: Emerging Market Shares (%), 2017-2022
Table 7.4 Pharma Contract Manufacturing Market: Emerging Market Shares (%), 2022-2028
Table 7.5 Chinese Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 7.6 Indian Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 7.7 Brazilian Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 7.8 Russian Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 7.9 South Korean Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 7.10 Turkey Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 7.11 Mexico Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), Annual Growth (%), CAGR (%), 2017-2028
Table 8.1 Catalent: Revenue ($bn), Annual Growth, CAGR, 2012-2017
Table 8.2 Lonza: Revenue (CHF bn), Annual Growth (%), CAGR (%), 2012-2017
Table 8.3 Teva: Revenue ($bn), Annual Growth (%), CAGR (%), 2012-2017
Table 8.4 Boehringer Ingelheim BioXcellence: Revenue (CHF bn), Annual Growth (%), CAGR (%), 2012-2017
Table 9.1 Pharma Contract Manufacturing Market Strengths and Weaknesses: CMO Perspective, 2017-2028
Table 9.2 Pharma Contract Manufacturing Market Opportunities and Threats: CMO Perspective, 2017-2028
Table 9.3 Pharma Contract Manufacturing Market: STEP Analysis, 2017-2028
Table 9.4 Selected Lyophilisation Service Providers
Table 9.5 Lyophilisation vs. Spray Drying: A Comparison
Table 9.6 Selected Orphan Drug Prices, 2014
Table 9.7 Selected Biological Manufacturing Expansion, 2013-2017
Table 9.8 Selected EU-Approved Biosimilars: Manufacturers and Companies, 2016
Table 9.9 Selected CMOs Growing Faster than the Market, 2007-2012
Table 9.10 Regional Market Expansion, 2010-2016
Table 10.1 Pharma Contract Manufacturing Market Strengths and Weaknesses: Client Perspective, 2017-2028
Table 10.2 Pharma Contract Manufacturing Market Opportunities and Threats: Client Perspective, 2017-2028
Table 10.3 Benefits and Risks to Outsourcing Pharma Manufacturing
Table 10.4 ICH Guidelines: Key Facts and Adoption, 2000-2014
Table 12.1 Pharma Contract Manufacturing Market: Revenue ($bn), CAGR (%), and Market Share (%) by Sector, 2017, 2022 and 2028
Table 12.2 Pharma Contract Manufacturing Market: Revenue ($bn), CAGR (%), and Market Share (%) by Region, 2017, 2022 and 2028
List of Figures
Figure 1.1 Global Pharmaceutical Contract Manufacturing Market Segmentation Overview, 2017
Figure 2.1 Selected Services Offered by CMOs
Figure 2.2 Major Trends Affecting Pharma Contract Manufacturing Revenue Growth, 2017-2028
Figure 3.1 Pharmaceutical Contract Manufacturing Market Revenue ($bn), 2012-2016
Figure 3.3 Pharma Contract Manufacturing: Market Share (%) by Sector, 2017
Figure 3.4 Pharmaceutical Contract Manufacturing Market: Overall Market Forecast, Revenue ($bn), 2017-2028
Figure 3.5 Pharma Contract Manufacturing Market: Shares (%), 2022
Figure 3.6 Pharma Contract Manufacturing Market: Shares (%), 2028
Figure 3.7 Pharma Contract Manufacturing Market: Drivers, 2017-2028
Figure 3.8 Pharma Contract Manufacturing Market: Restraints, 2017-2028
Figure 3.9 Other Services Submarket Forecast, Revenue ($bn), 2017-2028
Figure 4.1 Contract API Manufacturing Submarket Revenue ($bn), 2012-2016
Figure 4.2 Contract API Manufacturing Submarket: Segment Shares (%) by Sector, 2017
Figure 4.3 Contract API Manufacturing Submarket Forecast, Revenue ($bn), 2017-2028
Figure 4.4 Contract API Manufacturing Submarket: Segment Shares (%), 2022
Figure 4.5 Contract API Manufacturing Submarket: Segment Shares (%), 2028
Figure 4.6 Contract Generic API Manufacturing Submarket Forecast, Revenue ($bn), 2017-2028
Figure 4.7 Contract HPAPI Manufacturing Submarket Forecast, Revenue ($bn), 2017-2028
Figure 5.1 Contract FDF Manufacturing Submarket Revenue ($bn), 2012-2016
Figure 5.2 Contract FDF Manufacturing Submarket: Segment Shares (%) by Sector, 2017
Figure 5.3 Contract FDF Manufacturing Submarket Forecast, Revenue ($bn), 2017-2028
Figure 5.4 Contract FDF Manufacturing Submarket: Segment Shares (%), 2022
Figure 5.5 Contract FDF Manufacturing Submarket: Segment Shares (%), 2028
Figure 5.6 Contract Solid Dosage Manufacturing Submarket Forecast ($bn), 2017-2028
Figure 5.7 Contract Injectable Dosage Manufacturing Submarket Forecast, Revenue ($bn), 2017-2028
Figure 6.1 Pharma Contract Manufacturing Market: Share (%) by Region, 2017
Figure 6.2 Pharmaceutical Contract Manufacturing Market: Share (%) by Region, 2022
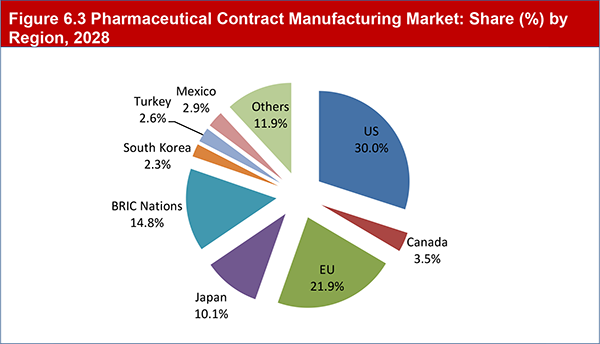
Figure 6.3 Pharmaceutical Contract Manufacturing Market: Share (%) by Region, 2028
Figure 6.4 US Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 6.4 Canada Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 6.5 EU5 Pharma Contract Manufacturing Market: Share (%) by Country, 2017
Figure 6.6 EU5 Pharma Contract Manufacturing Market: Share (%) by Country, 2022
Figure 6.7 EU5 Pharma Contract Manufacturing Market: Share (%) by Country, 2028
Figure 6.8 EU5 Pharma Contract Manufacturing Market, Revenue ($bn), 2017-2028
Figure 6.9 German Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 6.10 French Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 6.11 Italian Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 6.12 Spanish Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 6.13 UK Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 6.14 Japanese Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 7.1 Pharma Contract Manufacturing Market: Emerging Market Share (%) by Top Country, 2017
Figure 7.2 Pharma Contract Manufacturing Market: Emerging Market Share (%) by Top Country, 2022
Figure 7.3 Pharma Contract Manufacturing Market: Emerging Market Share (%) by Top Country, 2028
Figure 7.4 Pharma Contract Manufacturing Market: Emerging Market Share (%) by Top Country, 2017, 2022 and 2028
Figure 7.5 Chinese Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 7.6 Indian Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 7.7 Brazilian Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 7.8 Russian Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 7.9 South Korean Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 7.9 Turkey Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 7.10 Mexico Pharma Contract Manufacturing: Market Forecast, Revenue ($bn), 2017-2028
Figure 8.1 Catalent: Revenue ($bn), 2012-2017
Figure 8.2 Lonza: Revenue (CHF bn), 2012-2017
Figure 8.3 Teva: Revenue ($bn), 2012-2017
Figure 8.4 Boehringer Ingelheim BioXcellence: Revenue ($bn), 2012-2017
Figure 10.1 Overview of Approved ICH Manufacturing Guidelines
Figure 12.1 Pharma Contract Manufacturing Market: Revenue ($bn) by Sector, 2017, 2022 and 2028
Figure 12.2 Pharma Contract Manufacturing Market: Revenue ($bn) by Region, 2017, 2022 and 2028
AbbVie
Actavis
ADC Biotechnology
Aegerion Pharmaceuticals
Aenova Group
Aesica Pharma
Agensys
Agila Specialties
Ajinomoto Althea Inc
Alexion Pharmaceuticals
Almac Group
Althea Technologies
AMRI (Albany Molecular Research Inc)
API Corporation (APIC)
Aspen Pharmacare
Astellas
AstraZeneca
Banner Life Sciences
Banner Pharmacaps
Baring Private Equity Asia
Bausch & Lomb
Bayer Healthcare
Ben Venue Laboratories
Bend Research
Biogen Idec
BioIndustry Association (BIA)
Biotest
Blackstone Group
Boehringer Ingelheim
Boehringer Ingelheim BioXcellence
Bristol-Myers Squibb Company
Bushu Pharmaceuticals
Cambrex
Cambridge Major Laboratories
Catalent Pharma Solutions
Cedarburg Hauser
Cell Therapy Catapult
Celldex Therapeutics
Celltrion
Cenexi
Chemisch-Pharmazeutisches Laboratorium Ravensburg
Chemtrix
China FDA (CFDA)
Cipla
Cook Pharmica
CordenPharma
CTC Bio
Daito Pharmaceutical
Delpharm
Dishman Pharmaceuticals
DPx Fine Chemicals
DPx Holdings B.V.
Dr. Reddy’s Laboratories
DSP (DSM Sinochem Pharmaceuticals)
Eisai
Eli Lilly
Esteve Quimica
Euticals
Evonik Degussa
Famar
Fareva
FUJIFILM Diosynth Biotechnologies,
Gallus Biopharmaceutical, LLC.
G-CON
GEA Pharma-Systems
Genentech
GlaxoSmithKline (GSK)
Granules India
Haupt Pharma
Hexal
Hospira
Hospira One2One
ImmunoGen
Immunomedics
Indian Pharmaceutical Alliance
Innovent Biologics
IRIX Pharmaceuticals
Janssen
JK Pharmaceutical
Johnson & Johnson
Knowledge Transfer Network (KTN)
Lonza
Lupin
Marinopoulos Group
Matrix Laboratories
Medice
Medichem
Merck & Co.
Micron Technologies
Millennium
Mitsui & Co
Momenta Pharmaceuticals
Mylan
Neuland Laboratories
NICE Insight
NPS Pharmaceuticals
Nycomed
Orchid Chemicals & Pharmaceuticals
Oxford Biomedica
Patheon
Patheon Biologics
Pfizer
Pharmapak Technologies
Piramal Pharma Solutions
Progenics
Quintiles
Recepta Biopharma
Recipharm
Redwood Bioscience
Rentschler Biotechnologie
Roche
Royal DSM
SAFC
SafeBridge
Samsung Bioepis
Samsung BioLogics
Sandoz
Sanofi
Seattle Genetics
Shandong Xinhua
ShangPharma
Shire
Siegfried AG
Sigmar Italia
SMS Pharmaceuticals
Solvias AG
Speedel
Stada
Stem CentRx
Stevenage Bioscience Catalyst
Takeda
Temmler Group
Teva API
Thermo Fisher Scientific
UMN Pharma
UNIGEN
Valeant Pharmaceuticals
Valerion Therapeutics, LLC.
Vetter Pharma-Fertigung GmbH
Vivante GMP Solutions
West Pharmaceutical Services
WuXi PharmaTech
Zhangjiang Biotech & Pharmaceutical Base Company
Zhejiang Jiang Yuan Tang Biotechnology
List or Organizations Mentioned in the Report
Agência Nacional de Vigilância Sanitária (ANVISA)
Asociación Española de Fabricantes de Productos de Química Fina (AFAQUIM)
Associação Brasileira da Indústria Farmoquímica e de Insumos Farmacêuticos (ABIQUIFI)
Association of British Pharmaceutical Industry (ABPI)
Central Drugs Standard Control Organization (CDSCO)
Department of Health and Family Welfare
Development and Reform Commission (NDRC)
European Commission
European Medicines Agency (EMA)
Food and Drug Administration (US FDA)
Indian Drug Manufacturer’s Association (IDMA)
International Society of Pharmaceutical Engineering (IPSE)
Korea Food and Drug Administration (KFDA)
Korea Pharmaceutical Manufacturer’s Association (KPMA)
Medicines and Healthcare Products Regulatory Agency (MHRA)
Medicines Manufacturing Industry Partnership (MMIP)
Ministry of Health (MOH)
Ministry of Health, Labor and Welfare (MHLW)
Ministry of Industry and Information Technology (MIIT)
Pharmaceutical and Medical Devices Agency (PMDA)
World Health Organization (WHO)
Download sample pages
Complete the form below to download your free sample pages for Pharmaceutical Contract Manufacturing Market 2018-2028
Related reports
-

Global Ophthalmic Drugs Market Forecast 2018-2028
The global ophthalmic drugs market is expected to grow at a CAGR of 4.4% in the first half of the...
Full DetailsPublished: 26 June 2018 -

Global Biosimilar Monoclonal Antibodies Forecast 2018-2028
The global biosimilar monoclonal antibodies market is expected to reach $5.9bn in 2023 and is estimated to grow at a...
Full DetailsPublished: 27 February 2018 -

Top 25 Antibiotic Drugs Manufacturers 2018
Visiongain forecasts the antibiotic drugs market to increase to $ 43,841.7m in 2022. The market is estimated to grow at...
Full DetailsPublished: 12 June 2018 -

Vaccine Sales Market Forecast 2018-2028
The Global Vaccines Sales market was valued at $36.9 billion in 2017. This value will grow to $61.1bn in 2022...
Full DetailsPublished: 09 March 2018 -

Global Liquid Biopsy Market Forecast 2018-2028
Our 156-page report provides 144 tables, charts, and graphs. Read on to discover the most lucrative areas in the industry...
Full DetailsPublished: 24 April 2018 -

Drug Delivery Technologies Market Forecast 2018-2028
Our 188 page report provides 118 tables, charts, and graphs. Discover the most lucrative areas in the industry and the...
Full DetailsPublished: 01 February 2018 -

Global Anaesthesia Drugs Market 2018-2028
The global anaesthesia drugs market is expected to reach $10.5bn in 2022 and is estimated to grow at a CAGR...
Full DetailsPublished: 28 February 2018 -

Global Infusion Devices Market Forecast 2018-2028
The global infusion devices market was valued at $2.1bn in 2017 and is estimated to reach $3.9bn by 2028, growing...Full DetailsPublished: 16 August 2018 -

Pharma Leader Series: Top 25 Ophthalmic Drug Manufacturers 2018-2028
The global ophthalmic drugs market was valued at $23bn in 2017. The market was dominated by the Retinal Disorder drug...
Full DetailsPublished: 19 July 2018 -

Global Ophthalmic Devices Market 2018-2028
The global ophthalmic devices market is expected to grow at a CAGR of 5.5% in the first half of the...
Full DetailsPublished: 25 June 2018
Download sample pages
Complete the form below to download your free sample pages for Pharmaceutical Contract Manufacturing Market 2018-2028
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Latest Pharma news
Visiongain Publishes Drug Delivery Technologies Market Report 2024-2034
The global Drug Delivery Technologies market is estimated at US$1,729.6 billion in 2024 and is projected to grow at a CAGR of 5.5% during the forecast period 2024-2034.
23 April 2024
Visiongain Publishes Cell Therapy Technologies Market Report 2024-2034
The cell therapy technologies market is estimated at US$7,041.3 million in 2024 and is projected to grow at a CAGR of 10.7% during the forecast period 2024-2034.
18 April 2024
Visiongain Publishes Automation in Biopharma Industry Market Report 2024-2034
The global Automation in Biopharma Industry market is estimated at US$1,954.3 million in 2024 and is projected to grow at a CAGR of 7% during the forecast period 2024-2034.
17 April 2024
Visiongain Publishes Anti-obesity Drugs Market Report 2024-2034
The global Anti-obesity Drugs market is estimated at US$11,540.2 million in 2024 and is expected to register a CAGR of 21.2% from 2024 to 2034.
12 April 2024