Industries > Pharma > Biologics Market Trends and Forecasts 2018-2028
Biologics Market Trends and Forecasts 2018-2028
Protein Therapeutics, Monoclonal Antibodies, Fusion Proteins, Regenerative Medicines, Insulin, Other Recombinant Hormones, Plasma & Recombinant Coagulating Factors, Interferons, Enzyme Replacement, Stem Cell Therapies, Tissue Engineered Products, Gene Therapies
The global biologics market is estimated to reach $250bn in 2023. The market is expected to grow at a CAGR of 4.8% from 2018 to 2023. In 2017, the monoclonal antibodies submarket held 36.3% share of the global biologics market.
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in this sector.
In this brand new 303-page report you will receive 166 charts– all unavailable elsewhere.
The 303-page report provides clear detailed insight into the global biologics market. Discover the key drivers and challenges affecting the market.
By ordering and reading our brand-new report today you stay better informed and ready to act.
Report Scope
• Global Biologics Market outlook from 2018-2028
• Global Biologics Submarkets analysis and forecast from 2018-2028:
• Protein therapeutics, with sub-forecasting for insulin, other recombinant hormones, plasma and recombinant coagulating factors, interferons, enzyme replacement and other agents
• Monoclonal antibodies (mAbs)
• Fusion proteins
• Regenerative medicine, with sub-forecasting for stem cell treatment, tissue engineering and gene therapy
• Vaccines
• Analysis and forecast from 2018-2028 for selected leading biologics in the market:
• Lantus
• NovoLog/NovoRapid
• Humalog
• Avonex
• Rebif
• Humira
• Remicade
• Tysabri
• Herceptin
• Kadcyla
• Perjeta
• Enbrel
• Eylea
• OsteoCel Plus
• Trinity Evolution and Trinity Elite
• Apligraf
• Dermagraft
• IMLYGIC
• This report provides individual revenue forecasts from 2018-2028 for these national markets:
• The US
• Japan
• Germany
• France
• UK
• Italy
• Spain
• China
• India
• Russia
• Brazil
• Our study discusses the selected leading companies that are the major players in the biologics market:
• AbbVie
• Amgen
• AstraZeneca
• Bayer
• Eli Lilly
• GlaxoSmithKline (GSK)
• Johnson & Johnson
• Merck & Co., Inc.
• Novartis
• Pfizer
• Roche
• Sanofi S.A.
• This report discusses factors that drive and restrain this market. As well as opportunities and trends in this market.
• This report discusses the SWOT and STEP Analysis of the biologics market.
Visiongain’s study is intended for anyone requiring commercial analyses for the biologics market. You find data, trends and predictions.
Buy our report today Biologics Market Forecasts : Protein Therapeutics, Monoclonal Antibodies, Fusion Proteins, Regenerative Medicines, Insulin, Other Recombinant Hormones, Plasma & Recombinant Coagulating Factors, Interferons, Enzyme Replacement, Stem Cell Therapies, Tissue Engineered Products, Gene Therapies.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
1. Report Overview
1.1 Biologics Overview
1.2 Why you Should Read this Report
1.3 How this Report Delivers
1.4 Main Questions Answered by this Report
1.5 Who is this Report for?
1.6 Research and Analysis Methods
1.7 Frequently Asked Questions (FAQ)
1.8 Associated Visiongain Reports
1.9 About Visiongain
2. Market Dynamics
2.1 Drivers
2.1.1 Increasing Biologics Approvals
2.1.2. Rise in the Prevalence of Chronic Diseases
2.2 Restraints
2.2.1 Complex in Nature
2.2.2 High Cost
2.3 Opportunities
2.3.1 Strong Pipeline of biologics drugs
2.3.2 Emerging technology
2.4 Trends
2.4.1 Growing strategic partnerships in China
2.4.2 Outsourcing strategies
3. Introduction to the Biologics Market and Key Concepts
3.1 Biologics: Large and Complex Products
3.2 A Brief History of Biological Drug Development
3.3 Why are Biologics the Most Lucrative Products in the Global Pharmaceutical Market?
3.4 What are Biosimilars?
3.4.1 Brief History of Biosimilars
3.4.2 What are Interchangeable Biological Products and how do They Differ from Biosimilars?
3.4.3 Why Many Nations Oppose Automatic Substitution of Reference Biologics with Biosimilars
3.5 Marginal Cases
3.6 Vaccines Market
4. The Global Biologics Market 2018 - 2028
4.1 The Global Biologics Market 2018 - 2028
4.2 What Factors Will Drive Growth in the Biologics Market?
4.2.1 Ageing Population and the Rise of Chronic Disease
4.2.2 Biologics Constitute 19% of the Global Pharmaceutical Market - Further Launches Will Drive Market
4.3 Biosimilars - Both an Opportunity and a Threat
4.4 What Factors Will Restrain Growth in the Biologics Market?
4.4.1 High Costs in the Face of Declining National Healthcare Budgets
4.4.2 In Some Areas, Clinical Efficacy is not Superior Enough to Justify the Price Gap
4.4.3 Over $100bn worth of Biologic Patents due to Expire by 2020
4.4.4 Administration is Often not as Convenient
4.5 Biologics Market: Submarket Forecasts 2018 - 2028
5. Protein Therapeutics Submarket 2018 - 2028
5.1 Largest and Most Diverse Submarket
5.2 Proteins have been used Medicinally since the 19th Century
5.2.1 Recombinant DNA Technology - The Major Breakthrough in the use of Protein Drugs
5.3 Protein Therapeutics Submarket Forecast 2018 - 2028
5.4 Insulin Submarket 2018 - 2028
5.4.1 The First Protein Therapeutic
5.4.2 Around 430 Million People Expected to be Diagnosed with Diabetes by 2030
5.4.3 Insulin Submarket Forecast 2018 - 2028
5.4.4 Lantus: The Market Leading Drug by Far
5.4.4.1 Lantus Revenue Forecast 2018 - 2028
5.4.5 NovoLog/NovoRapid Revenue Forecast to 2028
5.4.6 Humalog Revenue Forecast to 2028
5.4.7 New Approvals and Pipeline
5.4.7.1 Tresiba (Novo Nordisk)
5.4.7.2 BIOD-123 (Biodel)
5.5 Biosimilar Insulin
5.5.1 Approved Insulin Biosimilars
5.5.2 Abasaglar/Basaglar/Insulin Glargine BS - The First Insulin Biosimilars to be Approved in Developed Markets. Delayed in the US
5.6 Other Recombinant Hormones: Erythropoietins, G-CSF and Human Growth Hormone
5.6.1 Erythropoietin
5.6.1.1 First-Generation Therapies Launched in the 1980s
5.6.1.2 Aranesp: Slowly Growing
5.6.1.3 Epogen: Increases in Selling Price are No Longer Enough to Ward off Decline
5.6.1.4 Procrit/Eprex: In Decline Since 2013
5.6.1.5 NeoRecormon and Mircera (Roche) Provides Competition for Market Leader Amgen
5.6.1.6 Safety Concerns for Erythropoietin-Stimulating Agents is Leading to Decreased Demand
5.6.1.7 The Challenge from Oral Therapies is Coming
5.6.1.8 Treating Anaemia in Patients with CKD - The Leading Use of EPO Therapies
5.6.1.9 Many Biosimilar Epoetins Approved Around the World
5.6.2 G-CSF
5.6.2.1 G-CSF: Discovered 1983. Recombinant Forms Available Since 1991
5.6.2.2 Amgen Leads the Market
5.6.2.3 Neupogen and Neulasta Revenues, and the Onpro Kit
5.6.2.4 Teva Aiming to Challenge Amgen’s Dominance
5.6.2.5 Facing Much Biosimilar Competition Around the World
5.6.3 Human Growth Hormone
5.6.3.1 Human Growth Hormone: First Extracted in 1958
5.6.3.2 Novo Nordisk Dominates Market
5.6.3.3 Novo Nordisk Aiming to Retain Dominance Through FlexPro Device and NN8640 Somatropin Candidate
5.6.3.4 Multiple Biosimilars Available - Omnitrope is Market Leader
5.6.3.5 Biosimilar Growth Hormones in Asia
5.6.4 Other Recombinant Hormones Submarket Forecast to 2028
5.7 Plasma and Recombinant Coagulating Factors
5.7.1 Types of Recombinant Factor
5.7.2 Clotting Factor Deficiency Diseases
5.7.3 Baxter/Baxalta: Entered the Bleeding Disorders Market in 1992, Markets Various Leading Products
5.7.3.1 Baxter: New Product Launches Include Rixubis and Obizur
5.7.4 Novo Nordisk: Committed to Remaining a Leading Company in the Bleeding Disorders Market
5.7.4.1 Novo Nordisk: Has filed for Approval for N9-GP and NovoEight is Doing Well
5.7.5 Bayer: Markets Kogenate, Kogenate FS, and has Received FDA Approval for Kovaltry
5.7.6 Limited Opportunities for Biosimilar Challenge
5.7.7 Plasma and Recombinant Coagulating Factors Submarket Forecast 2018 - 2028
5.8 Interferons
5.8.1 Interferons: Key Antiviral and MS Therapies Since the 1990s
5.8.2 Interferons for Treating Hepatitis: Its Influence is Decreasing
5.8.3 Competition from Oral Protease and Polymerase Inhibitors
5.8.3.1 Oral Therapies are Expensive
5.8.4 Avonex: The Leading Interferon Therapy
5.8.4.1 Avonex Revenue Forecast 2018 - 2028
5.8.5 Rebif: Merck Attempting to Differentiate Rebif from Other Interferon Therapies
5.8.5.1 Rebif Revenue Forecast 2018 - 2028
5.8.6 Betaseron
5.8.7 Plegridy: New Therapy with High Potential
5.8.8 Biosimilar Interferons: None Approved in Developed Markets, but Many Available in Emerging Nations
5.8.9 Interferon Submarket Forecast 2018 - 2028
5.9 Enzyme Replacement and Other Protein Therapies Submarket
5.9.1 Cerezyme: Genzyme’s Leading Enzyme Replacement Therapy
5.9.2 Myozyme and Lumizyme
5.9.3 Fabrazyme and Aldurazyme
5.9.4 Botulinum Toxin Brands Include Botox, Dysport and Xeomin
5.9.5 Other Protein Therapeutics Pipeline
5.9.5.1 Anthera Buys Sollpura from Eli Lilly, Phase 3 Results Expected Q4 2016
5.9.5.2 Multikine (CEL-SCI)
5.9.5.3 Vonapantiase (Proteon Therapeutics)
5.9.6 Enzyme Replacement and Other Protein Therapeutics Submarket Forecast 2018 - 2028
6. Monoclonal Antibodies Submarket 2018 - 2028
6.1 Monoclonal Antibodies Submarket: Scientific and Historical Background
6.1.1 Natural Antibodies: Key to the Immune System
6.1.2 From Serum Therapy to Monoclonal Antibodies
6.1.3 Humanising the mAb
6.1.4 MAbs have found Success in Treating Autoimmune Disorders, Cancers and Others Diseases - Great Potential for the Future
6.1.5 MAbs for All? Technologies Come Off Patent
6.2 Autoimmune mAbs
6.2.1 Past Approvals
6.2.2 Humira - The World’s Best-Selling Prescription Drug
6.2.2.1 AbbVie Plans to Drive Growth Through New Indications
6.2.2.2 Positive Opinion from CHMP for Treatment of Non-Infectious Uveitis
6.2.2.3 Recently Approved for Hidradenitis Suppurativa in the US, Europe, and Japan
6.2.2.4 Biosimilar Adalimumab: Patents Protect Humira in Developed Markets for Now
6.2.2.5 Humira Revenue Forecast 2018 - 2028
6.2.3 Remicade: Second Only to Humira
6.2.3.1 Has Defended Revenues Well but Now Has to Deal with Biosimilar Competition
6.2.3.2 Patent Dance in US
6.2.3.3 Biosimilar Infliximab: Multiple Launches
6.2.3.4 Remicade Revenue Forecast 2018 - 2028
6.2.4 Stelara: Blockbuster Status Well Established
6.2.4.1 Stelara Revenues 2015-2017
6.2.4.2 Orphan Drug Designation for paediatric Crohn’s Disease
6.2.5 Tysabri: Blockbuster Drug’s High Efficacy Offsets its Risks
6.2.5.1 Tysabri Revenue Forecast 2018 - 2028
6.2.6 Xolair: New Indication, and Revenue Breakdown
6.2.7 Actemra/RoActemra: Strong Growth in All Regions
6.2.8 Cimzia: Blockbuster Which has been Used by Over 90,000 Patients
6.2.9 Autoimmune mAbs Pipeline: RA is the Main Target
6.2.9.1 Sarilumab (REGN88/SAR153191) by Regeneron and Sanofi: BLA Accepted for Review and Decision Expected by November 2016
6.2.9.2 Sirukumab (CNTO-136) by J&J and GSK: Multiple Phase 3 Studies Around the World
6.2.9.3 Clazakizumab: Developed by Alder Biopharmaceuticals and BMS but now Licensed to Vitaeris
6.2.9.4 Tregalizumab (BT-061) by Biotest: AbbVie Backs out After Poor Results
6.2.9.5 Mavrilimumab by AstraZeneca: Approaching Phase 3
6.2.9.6 Zinbryta (daclizumab high-yield process) by Biogen and AbbVie: Positive Opinion from CHMP
6.2.9.7 Lebrikizumab by Roche: Undergoing Phase 3 After Positive Phase 2b Results
6.2.9.8 Sifalimumab (MEDI-545) by AstraZeneca: Hasn’t Made the Cut for Phase 3
6.2.9.9 Anifrolumab by AstraZeneca
6.3 Oncology mAbs
6.3.1 Past Approvals
6.3.2 Rising Incidence of Cancer Will Drive Demand
6.3.3 Rituxan: The World’s Leading Anti-Cancer mAb
6.3.4 Biosimilar Rituximab: Two Candidates Await Approval in Europe
6.3.5 Gazyva: Roche’s Successor to Rituxan, Will Help to Retain Revenues in Face of Biosimilar Competition
6.3.5.1 Approval for Follicular Lymphoma and Being Investigated for Other Indications
6.3.6 Avastin: Growing under CER, Through New Indications
6.3.6.1 Controversy over Off-Label Avastin use: Roche is Backed up by Incidents in India, and Opinion of EFPIA
6.3.6.2 Biosimilar Bevacizumab: Late Patent Expiry is a Blessing for Roche, Although Various Biosimilars in Development
6.3.7 Herceptin: Breast Cancer Blockbuster
6.3.7.1 Pricing Challenges in Emerging Markets, and 2016 Revenues
6.3.7.2 Roche Builds Franchise of Anti-HER2 mAbs Around Herceptin
6.3.7.3 Herceptin Revenue Forecast 2018 - 2028
6.3.7.4 Biosimilar Trastuzumab
6.3.8 Kadcyla
6.3.8.1 Mixed Results from Different Clinical Trials
6.3.8.2 Kadcyla Sales Forecast 2018 -2028
6.3.9 Perjeta
6.3.9.1 Positive Results from Different Clinical Trials
6.3.9.2 Perjeta Revenue Forecast 2018 - 2028
6.3.10 Yervoy: Bristol-Myers Squibb’s Leading Anti-Cancer mAb, KeepsHigh Revenues, but with Decline over 2015
6.3.11 Opdivo: Big Potential for Bristol-Myer Squibb’s PD-1 Inhibitor
6.3.11.1 Good News and Bad News for NSCLC from CheckMate Trials
6.3.11.2 Cyramza (Eli Lilly): Approved in Three Additional Indications Since its First Approval in April 2014
6.3.12 Oncology mAbs: New Approvals and Pipeline
6.3.12.1 Empliciti (Elotuzumab) by Bristol-Myers Squibb and AbbVie): Recently Approved
6.3.12.2 Avelumab: Pfizer and Merck KGaA Team Up to Develop Their Own Anti-PD mAb
6.3.12.3 Unituxin (United Therapeutics) Receives FDA Approval March 2015
6.3.12.4 SAR3419 (coltuximab ravtansine) (anti-CD19, Sanofi)
6.4 Monoclonal Antibodies Submarket Forecast 2018 - 2028
7. Fusion Proteins Submarket 2018 - 2028
7.1 Scientific and Historical Background
7.2 Differentiating Fusion Proteins
7.3 Enbrel: The First FP to be Approved, in 1998
7.3.1 Gradually Approved for Additional Indications
7.3.2 Enbrel Granted Extended Patent Protection in the US, Patent has Expired in EU
7.3.3 Multiple Biosimilars Available in Emerging Markets
7.3.4 Enbrel Revenue Forecast 2018 - 2028
7.4 Eylea: Blockbuster FP Treatment for Wet AMD
7.4.1 Eylea Revenue Forecast 2018 - 2028
7.5 Orencia: Grows Blockbuster Revenues for Second Year in a Row
7.6 Nplate: Generates Half a Billion in Revenues for Amgen
7.7 Eloctate: Approved June 2014, Fulfils Unmet Need in the Haemophilia Community
7.8 Nulojix: Bristol-Myers Squibb’s Therapy for Preventing Organ Rejection
7.9 Arcalyst by Regeneron Pharmaceuticals: For Very Rare Conditions
7.10 Fusion Proteins Pipeline
7.10.1 Trebananib (Amgen)
7.10.2 Sotatercept by Acceleron Pharma and Celgene Corporation - Currently Undergoing Eight Clinical Trials
7.10.3 Blisibimod (Anthera Pharmaceuticals) for the Treatment of Various Autoimmune Diseases, in Late Stage Development
7.11 Fusion Protein Submarket Forecast to 2018 - 2028
8. Regenerative Medicines Submarket 2018 - 2028
8.1 Regenerative Medicines Market Breakdown
8.2 Regenerative Medicines Submarket Forecast, 2018 - 2028
8.3 Stem Cell Therapies Submarket, 2018 - 2028
8.3.1 Stem Cells: Scientific Breakdown
8.3.2 Stem Cell Therapies Submarket Breakdown
8.3.3 Haematopoietic Stem Cell Transplantation: Over 95,000 Carried out in 2016
8.3.3.1 From Procedures to Products: Cord Blood Stem Cell Approvals
8.3.4 Osteocel Plus: The Leading Stem Cell Orthobiologic
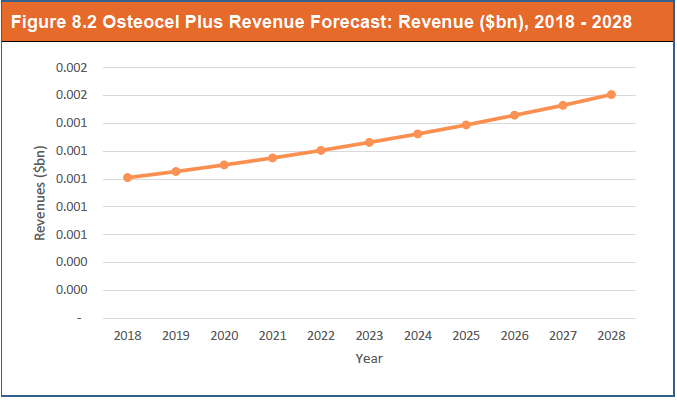
8.3.4.1 Osteocel Plus Revenue Forecast 2018 - 2028
8.3.5 Trinity Evolution and Trinity Elite
8.3.5.1 Trinity Evolution and Trinity Elite Revenue Forecast to 2028
8.3.6 MSC-100-IV (Previously Known as Prochymal): World’s First Approved Stem Cell Drug Outside of South Korea
8.3.6.1 An Important Role to Play in the Future of HSCT, and also Wins Approval in Japan through Partner
8.3.7 CARTISTEM (MEDIPOST): The World’s First Allogeneic Stem Cell Drug
8.3.8 Hearticellgram-AMI (PharmaCell B.V.): One of the First Approved Cardiovascular Stem Cell Treatments in the World
8.3.9 Stem Cell Therapies Pipeline
8.3.9.1 Cx601 by TiGenix: Marketing Authorisation Application has been Submitted to EMA
8.3.9.2 CardiAMP (BioCardia) for Heart Failure: Undergoing Phase 3 Trial and has Received Grant from MSCRF
8.3.9.3 NurOwn (BrainStorm Cell Therapeutics): Neurotrophic Factor -Releasing Stem Cells for ALS in Phase 2 and Due to end Soon
8.3.9.4 Agenmestencel-T (Apceth): Phase 1/2 Due to end Soon
8.3.10 Stem Cell Therapies Submarket Forecast 2018 - 2028
8.4 Tissue Engineering Therapies Submarket 2018 - 2028
8.4.1 Tissue Engineering – In Vitro Manipulation for Therapeutic Purposes
8.4.2 Current Status of the Market
8.4.3 Apligraf: The Leading Product
8.4.3.1 Apligraf Revenue Forecast 2018 - 2028
8.4.4 Dermagraft: Organogenesis Further Strengthens Position Through this 2014 Acquisition
8.4.4.1 Dermagraft Revenue Forecast 2018 - 2028
8.4.5 ReCell
8.4.6 MySkin and CyroSkin
8.4.7 Tissue Engineering Therapies Pipeline
8.4.7.1 NeoCart by Histogenics: Undergoing Phase 3 Trial for Knee Cartilage Repair
8.4.7.2 Extracorporeal Bio-Artificial Liver Therapy by Vital Therapies
8.4.7.3 StrataGraft by Stratatech Corporation Demonstrates Positive Top-Line Results
8.5 Gene Therapies Submarket 2018 - 2028
8.5.1 Gene Therapy: Historical and Scientific Background
8.5.2 Harnessing Infectivity: Viral Vectors in Gene Therapy
8.5.3 Approved Gene Therapy Products
8.5.3.1 Gendicine: The World’s First Commercial Gene Therapy Product
8.5.3.2 Oncorine: First Oncolytic Viral Therapy
8.5.3.3 Neovasculgen: Russia’s First Gene Therapy
8.5.3.4 Neovasculgen: Drop in Revenues in 2015, but HSCI Predicts Notable Increases as Product is Added to VED List
8.5.3.5 Glybera: The First Gene Therapy for Western Markets, Although Has Abandoned Hope of Receiving FDA-Approval
8.5.4 IMLYGIC
8.5.4.1 IMLYGIC: Clinical Trials and Development Efforts
8.5.4.2 IMLYGIC: Revenue Forecast 2018 - 2028
8.5.5 Gene Therapies Pipeline
8.5.5.1 AAV2-hRPE65v2 (Spark Therapeutics): Undergoing Phase 3 Trial
8.5.5.2 AdV-tk/ProstAtak (Advantagene): Phase 3
8.5.5.3 Collategene (beperminogene perplasmid, AMG0001) - (AnGes MG/Vical)
9. Leading National Markets for Biologics 2018 - 2028
9.1 Regional Forecasts for the Global Biologics Market to 2028
9.2 US Biologics Market 2018 - 2028
9.2.1 The Major National Market
9.2.2 Value-Based Pricing in the US
9.2.3 Regulation of Biologics in the US
9.2.4 US Biosimilars Market
9.2.4.1 FDA Finalises Biosimilar Guidelines and Biosimilar Naming
9.2.5 US Biologics Market Forecast 2018 - 2028
9.3 EU5 Biologics Market 2018 - 2028
9.3.1 Current Composition and Future Outlook
9.3.2 European Biosimilar Market
9.3.2.1 History of EMA Guidelines and Updates
9.3.3 EU5 Market Forecast by Nation 2018 - 2028
9.4 Japanese Biologics Market 2018 - 2028
9.4.1 Current Status of the Japanese Biologics Market
9.4.2 Japanese Biosimilar Market
9.4.3 Japanese Biologics Market Forecast 2018 - 2028
9.5 BRIC National Biologic Markets 2018 - 2028
9.5.1 BRIC Markets Overview
9.5.2 BRIC Biologic Market Forecast 2018 - 2028
9.5.2.1 Unique Challenges Faced by the Biologics Industry have led to Current Composition of the BRIC Biologic Market
9.5.2.2 Future Outlook
9.5.2.3 BRIC Nation Market Shares in the Global Biologics Market Will Nearly Double During the Forecast Period
9.5.3 Brazil: Disproportionately High Government Spending on Biologics
9.5.3.1 Brazilian Government Eager to Promote Domestic Biologic and Biosimilar Development
9.5.4 Russia: Another Government Which is Eager to Increase Domestic Biological Drug Development
9.5.5 India: Biologics Market Behind that of Other BRIC Nations, but Biosimilars Submarket Thriving
9.5.5.1 CDSCO Guidelines Released in 2012
9.5.6 China: Expected to be the Largest BRIC Market for Biologics by 2020
9.5.6.1 The Largest National Biosimilar Market in the World
10. Qualitative Analysis of the Biologics Market and Industry
10.1 SWOT Analysis of the Global Biologics Market and Industry
10.1.1 Strengths
10.1.2 Opportunities
10.1.3 Weaknesses
10.1.4 Threats
10.2 STEP Analysis of the Global Biologics Market and Industry
10.2.1 Social Factors
10.2.2 Technological Developments
10.2.3 Economic Pressures
10.2.4 Political Issues
11. Company Profiles
11.1 AbbVie Inc.: Profile
11.1.1 AbbVie Inc.: Company Overview
11.2 Amgen: Profile
11.2.1 Amgen: Company Overview
11.3 AstraZeneca PLC: Profile
11.3.1 AstraZeneca PLC: Company Profile
11.4 Bayer AG: Profile
11.4.1 Bayer AG: Profile
11.5 Eli Lilly: Profile
11.5.1 Eli Lilly: Company Overview
11.6 F. Hoffmann-La Roche Ltd.: Profile
11.6.1 F. Hoffmann-La Roche Ltd.: Company Overview
11.7 GlaxoSmithKline Plc.: Profile
11.7.1 GlaxoSmithKline Plc.: Company Overview
11.8 Johnson & Johnson Services, Inc.: Profile
11.8.1 Johnson & Johnson Services, Inc.: Company Overview
11.9 Merck & Co., Inc.: Profile
11.9.1 Merck & Co., Inc.: Company Overview
11.10 Novartis AG: Profile
11.10.1 Novartis AG: Profile
11.11 Pfizer Inc.: Profile
11.11.1 Pfizer Inc.: Company Overview
11.12 Sanofi S.A.: Profile
11.12.1 Sanofi S.A: Company Overview
12. Conclusions
12.1 Biologics Market to Achieve Steady Growth Throughout the Forecast Period
12.2 Changes in Market Composition: mAbs to Become Leading Submarket
12.3 The Emergence of Biosimilars
12.4 Challenges for the Market
Appendices
Visiongain Report Sales Order Form
Associated Visiongain Reports
About Visiongain
Visiongain report evaluation form
List of Tables
Table 3.1 First Approvals for Recombinant Protein Therapies, 1982-1993
Table 4.1 Global Biologics Market Forecast: Revenues ($bn), AGR (%), CAGR (%), 2018-2023
Table 4.2 Global Biologics Market Forecast: Revenues ($bn), AGR (%), CAGR (%), 2023-2028
Table 4.3 Patent Status of Selected Leading Biologics
Table 4.4 Submarkets within the Biologics Market, Forecast: Revenues ($bn), AGR (%), CAGR (%), Market Share (%), 2018-2023
Table 4.5 Submarkets within the Biologics Market, Forecast: Revenues ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 5.1 First Approvals for Recombinant Protein Therapies,1982-1993
Table 5.2 Protein Therapeutics Submarket Forecast: Revenues ($bn), AGR (%), CAGR (%), 2018-2023
Table 5.3 Protein Therapeutics Submarket Forecast: Revenues ($bn), AGR (%), CAGR (%), 2023-2028
Table 5.4 Diabetes Prevalence in Leading National Markets, 2017
Table 5.5 Insulin Submarket Forecast: Revenues ($bn), AGR (%), CAGR (%), 2018-2023
Table 5.6 Insulin Submarket Forecast: Revenues ($bn), AGR (%), CAGR (%), 2023-2028
Table 5.7 Lantus Revenue Forecast: Revenues ($bn), AGR (%), CAGR (%), 2018-2023
Table 5.8 Lantus Revenue Forecast: Revenues ($bn), AGR (%), CAGR (%),2023-2028
Table 5.9 NovoLog/NovoRapid Revenue Forecast: Revenues ($bn), AGR (%), CAGR (%), 2018-2023
Table 5.10 NovoLog/NovoRapid Revenue Forecast: Revenues ($bn), AGR (%), CAGR (%), 2023-2028
Table 5.11 Humalog Revenue Forecast: Revenues ($bn), AGR (%), CAGR (%),2018-2023
Table 5.12 Humalog Revenue Forecast: Revenues ($bn), AGR (%), CAGR (%),2023-2028
Table 5.13 Selected Insulin Biosimilars which have been Approved and Launched
Table 5.14 Selected EPO Biosimilars Approved in India
Table 5.15 European Biosimilar Approvals: G-CSF
Table 5.16 Selected Filgrastim Biosimilars Approved Worldwide, 2016
Table 5.17 Other Recombinant Hormones Submarket Forecast: Revenues ($bn), AGR (%), CAGR (%), 2018-2023
Table 5.18 Other Recombinant Hormones Submarket Forecast: Revenues ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 5.19 Selected Coagulating Factors Significant in Segment
Table 5.20 Plasma and Recombinant Coagulating Factors Submarket Forecast: Revenues ($bn), AGR (%), CAGR (%), 2018-2023
Table 5.21 Plasma and Recombinant Coagulating Factors Submarket Forecast: Revenues ($bn), AGR (%), CAGR (%),2023-2028
Table 5.22 Selected Approved Branded Interferon Therapies
Table 5.23 Avonex Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 5.24 Avonex Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), to 2028
Table 5.25 Rebif Revenue Forecast: Revenue ($m), AGR (%), CAGR (%), 2018-2023
Table 5.26 Rebif Revenue Forecast: Revenue ($m), AGR (%), CAGR (%), 2023-2028
Table 5.27 Selected Interferon Alfa Biosimilars Approved Worldwide, 2016-2017
Table 5.28 Selected Interferon Beta Biosimilars Approved Worldwide, 2016-2017
Table 5.29 Interferon Submarket Forecast: Revenues ($bn), AGR (%), CAGR (%), 2018-2023
Table 5.30 Interferon Submarket Forecast: Revenues ($bn), AGR (%), CAGR (%), 2023-2028
Table 5.31 Enzyme Replacement and Other Protein Therapeutics Submarket Forecast: Revenues ($bn), AGR (%), CAGR (%), 2018-2023
Table 5.32 Enzyme Replacement and Other Protein Therapeutics Submarket Forecast: Revenues ($bn), AGR (%), CAGR (%),2023-2028
Table 6.1 Regions of an Antibody
Table 6.2 Natural Antibody Mechanisms of Action
Table 6.3 Selected Biosimilar Adalimumab Candidates
Table 6.4 Humira Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 6.5 Humira Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 6.6 Remicade Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 6.7 Remicade Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%),2023-2028
Table 6.8 Stelara Revenue 2015-2017:
Revenues ($bn), AGR (%)
Table 6.9 Tysabri Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 6.10 Tysabri Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 6.11 Xolair Revenue Breakdown (between Roche and Novartis): Revenue ($bn), AGR (%), 2016-2017
Table 6.12 Selected Ongoing Lebrikizumab Clinical Trials
Table 6.13 Selected Ongoing Anifrolumab Clinical Trials
Table 6.14 Herceptin Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%),2018-2023
Table 6.15 Herceptin Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%),2023-2028
Table 6.16 Kadcyla Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%),2018-2023
Table 6.17 Kadcyla Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%),2023-2028
Table 6.18 Perjeta Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 6.19 Perjeta Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 6.20 FDA-Approved Indications for Cyramza
Table 6.21 Monoclonal Antibodies Submarket Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 6.22 Monoclonal Antibodies Submarket Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 7.1 Amgen’s US Patents and Expiry Dates for Enbrel
Table 7.2 Enbrel Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 7.3 Enbrel Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%),2023-2028
Table 7.4 Eylea Revenue Breakdown 2016-2017: Regeneron and Bayer
Table 7.5 Eylea Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 7.6 Eylea Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 7.7 Bristol-Myers Squibb’s pipeline developments for Orencia
Table 7.8 Fusion Proteins Submarket Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 7.9 Fusion Proteins Submarket Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 8.1 Regenerative Medicines Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 8.2 Regenerative Medicines Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 8.3 Potency and Source of Stem Cells
Table 8.4 Osteocel Plus Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 8.5 Osteocel Plus Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 8.6 Trinity Evolution and Trinity Elite Revenue Forecast: Revenue ($m), AGR (%), CAGR (%), 2018-2023
Table 8.7 Trinity Evolution and Trinity Elite Revenue Forecast: Revenue ($m), AGR (%), CAGR (%), 2023-2028
Table 8.8 Stem Cell Therapies Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 8.9 Stem Cell Therapies Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 8.10 Apligraf Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 8.11 Apligraf Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 8.12 Dermagraft Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 8.13 Dermagraft Revenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2023-2028
Table 8.14 IMLYGICRevenue Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2023
Table 8.15 IMLYGICRevenue Forecast: Revenue ($bn), AGR (%), CAGR (%),
2023-2028
Table 9.1 Regional Forecasts for the Global Biologics Market: Revenues ($bn), AGR (%), CAGR (%), Market Share (%), 2018-2023
Table 9.2 Regional Forecasts for the Global Biologics Market: Revenues ($bn), AGR (%), CAGR (%), Market Share (%), 2023-2028
Table 9.3 US Biologics Market: Revenues ($bn), AGR (%), CAGR (%), Global Market Share (%), 2018-2023
Table 9.4 US Biologics Market: Revenues ($bn), AGR (%), CAGR (%), Global Market Share (%), 2023-2028
Table 9.5 European Biosimilar Approvals, 2006-2017
Table 9.6 EU5 Biologics Market: Revenues ($bn), AGR (%), CAGR (%), EU5 Market Share (%), 2018-2023
Table 9.7 EU5 Biologics Market: Revenues ($bn), AGR (%), CAGR (%), EU5 Market Share (%), 2023-2028
Table 9.8 Japanese Biologics Market: Revenues ($bn), AGR (%), CAGR (%), Global Market Share (%), 2018-2023
Table 9.9 Japanese Biologics Market: Revenues ($bn), AGR (%), CAGR (%), Global Market Share (%), 2023-2028
Table 9.10 BRIC Biologics Market: Revenues ($bn), AGR (%), CAGR (%), BRIC Market Share (%), 2018-2023
Table 9.11 BRIC Biologics Market: Revenues ($bn), AGR (%), CAGR (%), BRIC Market Share (%), 2023-2028
Table 11.1 AbbVie, Inc.: Profile
Table 11.2 AbbVie, Inc.: Key Developments
Table 11.3 Amgen: Profile
Table
Table 11.4 Amgen: Key Developments
Table 11.5 AstraZeneca PLC: Profile
Table 11.6 AstraZeneca PLC: Key Developments
Table 11.7 Bayer AG: Profile
Table 11.8 Eli Lilly: Profile
Table 11.9 Eli Lilly: Key Developments
Table 11.10 Roche: Profile
Table 11.11 Roche: Key Developments
Table 11.12 GSK: Profile
Table 11.13 Johnson & Johnson: Profile
Table 11.14 Johnson & Johnson: Profile
Table 11.15 Merck & Co., Inc.: Profile
Table 11.16 Merck & Co., Inc.: Key Developments
Table 11.17 Novartis: Profile
Table 11.18 Novartis: Key Developments
Table 11.19 Pfizer: Profile
Table 11.20 Pfizer: Key Developments
Table 11.21 Sanofi: Profile
Table 11.22 Sanofi: Key Developments
List of Figures
Figure 1.1 Forecasted Submarkets of the Biologics Market
Figure 4.1 Global Biologics Market Forecast: Revenues ($bn), 2018 - 2028
Figure 4.2 Submarkets within the Biologics Market Forecast: Revenues ($bn),2017-2028
Figure 5.1 Protein Therapeutics Submarket Forecast: Revenues ($bn), 2018 - 2028
Figure 5.2 Diabetes Prevalence in Leading National Markets, 2017
Figure 5.3 Insulin Submarket Forecast: Revenues ($bn), 2018 - 2028
Figure 5.4 Lantus Revenue Forecast: Revenues ($bn), 2018 - 2028
Figure 5.5 NovoLog/NovoRapid Revenue Forecast: Revenues ($bn), 2018 - 2028
Figure 5.6 Humalog Revenue Forecast: Revenues ($bn), 2018 - 2028
Figure 5.7 Other Recombinant Hormones Submarket Forecast: Revenues ($bn), 2018 - 2028
Figure 5.8 Plasma and Recombinant Coagulating Factors Submarket Forecast: Revenues ($bn), 2018 - 2028
Figure 5.9 Avonex Submarket Forecast: Revenues ($bn), 2018 - 2028
Figure 5.10 Rebif Revenue Forecast: Revenue ($bn), 2018 - 2028
Figure 5.11 Interferon Submarket Forecast: Revenues ($bn), 2018 - 2028
Figure 5.12 Enzyme Replacement and Other Protein Therapeutics Submarket Forecast: Revenues ($bn), 2018 - 2028
Figure 6.1 Humira Revenue Forecast: Revenue ($bn), 2018 - 2028
Figure 6.2 Remicade Revenue Forecast: Revenue ($bn), 2018 - 2028
Figure 6.3 Stelara Revenue 2015-2017, Revenue ($bn)
Figure 6.4 Tysabri Revenue Forecast: Revenue ($m), 2018 - 2028
Figure 6.5 Herceptin Revenue Forecast: Revenue ($bn), 2018 - 2028
Figure 6.6 Kadcyla Revenue Forecast: Revenue ($m), 2018 -2028
Figure 6.7 Perjeta Revenue Forecast: Revenue ($bn), 2018 - 2028
Figure 6.8 Monoclonal Antibodies Submarket Forecast: Revenue ($bn), 2018 - 2028
Figure 7.1 Enbrel Revenue Forecast: Revenue ($bn), 2018 - 2028
Figure 7.2 Eylea Revenue Forecast: Revenue ($bn), 2018 - 2028
Figure 7.3 Fusion Proteins Forecast: Revenue ($bn), 2018 - 2028
Figure 8.1 Regenerative Medicines Market Forecast: Revenue ($bn), 2018 - 2028
Figure 8.2 Osteocel Plus Revenue Forecast: Revenue ($bn), 2018 - 2028
Figure 8.3 Trinity Evolution and Trinity Elite Revenue Forecast: Revenue ($m), 2018 - 2028
Figure 8.4 Stem Cell Therapies Market Forecast: Revenue ($bn), 2018 - 2028
Figure 8.5 Apligraf Revenue Forecast: Revenue ($bn), 2018 - 2028
Figure 8.6 Dermagraft Revenue Forecast: Revenue ($bn), 2018 - 2028
Figure 8.7 IMLYGIC Revenue Forecast: Revenue ($bn), 2018 - 2028
Figure 9.1 Regional Breakdown of Global Biologics Market: Revenues ($bn), Market Share (%), 2017
Figure 9.2 Regional Breakdown of Global Biologics Market: Revenues ($bn), Market Share (%), 2028
Figure 9.3 Regional Forecasts for Global Biologics Market: Revenues ($bn),to 2028
Figure 9.4 US Biologics Market: Revenues ($bn), 2018 - 2028
Figure 9.5 EU5 Market Breakdown: Market Share (%), 2017
Figure 9.6 EU5 Biologics Market: Revenues ($bn), 2018 - 2028
Figure 9.7 Japanese Biologics Market: Revenues ($bn), 2018 - 2028
Figure 9.8 Biologics Market Breakdown: Revenues ($bn), Market Share (%), 2017
Figure 9.9 BRIC Biologics Market: Revenues ($bn), 2018 - 2028
Figure 9.10 Biologics Market Breakdown: Revenues ($bn), Market Share (%), 2028
Figure 10.1 SWOT Analysis of Biologics Market and Industry
Figure 10.2 STEP Analysis of Biologics Market and Industry
Figure 12.1 Global Biologics Market Forecast: Revenues ($bn), Annual Growth Rate (%), 2018 - 2028
3SBio
AbbVie
Ablynx
Acceleron Pharma
Accord Healthcare
Aché
Actavis
Adaptive Biotechnologies Corporation
Advanced BioHealing
Advantagene
Advaxis, Inc.
Afferent Pharmaceuticals
Alder Biopharmaceuticals
Alector
Allergan
Alteogen
Amega Biotech
Amgen
AM‐Pharma B.V.
Anacor Pharmaceuticals, Inc.
AnGes
Anthera Pharmaceuticals
Apceth
Apeiron Biologics
Apotex
Argenx
ARMO BioSciences, Inc.
Aryogen
Aspen Global Incorporated
Astellas Pharma
AstraZeneca
AurKa Pharma, Inc.
Aventis
Avita Medical
Bamboo Therapeutics, Inc.
Banner Alzheimer's Institute
Basilea Pharmaceutica Ltd.
Baxalta
Bayer
Beijing Four Rings
Beijing SL Pharmaceutical
Benda Pharmaceuticals
Biocad
BioCardia
Biocon
Biodel
Biogen
Bionovis
Biopartners
BioPharma
Biosidus
Biotest
Bioton
BioXpress Therapeutics
Boehringer Ingelheim
BrainStorm Cell Therapeutics
Bristol-Myers Squibb (BMS)
Cambridge Antibody Technology
cCAM Biotherapeutics
CCL Pharmaceuticals
Celgene Corporation
Celltrion
CEL-SCI
Centocor Ortho Biotech
Children’s Oncology Group
CHMP
Chugai
CinnaGen
ClinImmune Labs
CSL Behring
CT Arzneimittel
CureVac AG
Cystic Fibrosis Foundation Therapeutics Inc
CytomX Therapeutics
Daiichi Sankyo Company, Limited
Dako
Dendreon
Dezima Pharma B.V.
DNAtrix
Dong-A Pharmaceutical
Dr. Reddy's Laboratories
Dynavax Technologies Corporation
Eisai Co., Ltd.
Eli Lilly and Company
Emcure Pharmaceuticals
EMS
Epirus
Europharm
Evotec
FDA
FibroGen
FierceBiotech
Finox Biotech
Flatiron Health
Foundation Medicine, Inc.
Fuji Pharma
Galencia
Gan & Lee
GE Healthcare
Genentech
Gennova
GenSci
Genzyme
Gilead Sciences
GlaxoSmithKline (GSK)
Hangzhou Jiuyuan Gene Engineering Co.
Hanwha Chemical
Harpoon Therapeutics
Harrisvaccines Inc.
Heptares Therapeutics
Hexal
Hindustan Antibiotic
Histogenics
Hospira, Inc.
Human Genome Sciences
Humana Inc.
Hypermarcas
IBM Watson Health
ICU Medical Inc.
Ignyta, Inc.
Immatics Biotechnologies GmbH
Immune Design
Immunex Corporation
Immunocore Limited
Incyte Corporation
Innovare R&D
Intas Pharmaceuticals
IOmet Pharma
Ipsen
Isis Pharmaceuticals, Inc.
Janssen
JingYuan Bio
Johnson & Johnson
Kite Pharma
Kyowa Hakko Kirin
Laboratorios Delta
LG Life Sciences
LifeSouth Community Blood Centers
Lonza
Luye Pharma Group, Ltd.
M2Gen
MacroGenics, Inc.
Massachusetts General Hospital
Medice
MEDICE Arzneimittel Pütter
MedImmune
MEDIPOST
Medivation, Inc.
Medtronic
Merck & Co., Inc.
Merck KGaA
Merz Pharma
Mirati Therapeutics, Inc.
Mitsubishi Tanabe
Mochida Pharmaceutical
Moderna Therapeutics
Mylan
NanoString Technologies, Inc.
NGM Biopharmaceuticals, Inc.
Nippion Kayaku
Northwestern University
Novartis
Novo Nordisk
NuVasive
Oanda
Organogenes
OrLife Bio
Orthofix
Orygen
Peregrine Pharmaceuticals, Inc.
Pfizer
Pharmacyclics
Pharmicell
Pharmstandard
Plexxikon Inc.
Porbiomed
Portola Pharmaceuticals Inc.
Premier Inc.
Principia
Probiomed
Protein Sciences
Proteon Therapeutics
Qilu Pharmaceutical
Ranbaxy
Ratiopharm
Redvax GmbH
Regeneron Pharmaceuticals, Inc.
Regenerys
Reliance Life Sciences
Rentschler Biotechnologie.
Rigontec
Roche
Samsung Bioepis
Sandoz
Sangamo Therapeutics, Inc.
Sanofi
Schering-Plough
SciGen
Shandong Kexing Pharma
Shanghai CP Guojian Pharmaceutical
Shanghai Fosun
Shanghai Sunway Biotech
Shantha Biotechnics
Shenzhen SiBiono GeneTech
Shire
Sicor Biotech
Sigilon Therapeutics
Simcere Pharmaceutical Group
Smith and Nephew
Spark Therapeutics
Stada
Stemcentrx
Stragen Pharma
Stratatech Corporation
Swiss Re
Takeda Pharmaceutical Company Limited
TESARO, Inc.
TetraLogic Pharmaceuticals Corporation
Teva
Tianjin Hualida Biotechnology
TiGenix
Tonghua Dongbao
Trophos
Turnstone Biologics
UCB
União Química
UniQure
Valeant Pharmaceuticals
Vertex Pharmaceuticals
Viralytics Limited
Vital Therapies Inc.
Voyager Therapeutics
Wockhardt
Wyeth-Ayerst Laboratories
Xencor, Inc.
Xiamen Amoytop Biotech
XO1 Limited
Zenotech
ZS Pharma
Zuventus
Zydus Biovation
Zydus Cadila
Organizations Mentioned in the Report
Agência Nacional de Vigilância Sanitária (ANVISA)
Australia's Walter and Eliza Hall Institute
Boston Children's Hospital
Center for Disease Prevention and Control (CDC)
Central Drugs Standard Control Organisation
China Food and Drug Administration
Duke University School of Medicine
EMA (European Medicines Agency)
European Commission (EC)
European Federation of Pharmaceutical Industries and Associations
Harvard University
Human Stem Cells Institute
International Myeloma Foundation
Johns Hopkins
Musculoskeletal Transplant Foundation (MTF)
National Cancer Institute
New York Blood Center
NHS
NICE
Rice University
Saudi Food and Drug Authority
SSN Cardinal Glennon Children’s Medical Center
Stanford University
The European Generic medicines Association (EGA)
The European Group for Blood and Marrow Transplantation (EBMT)
The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR)
The University of Manchester
The University of Texas
The University of Texas MD Anderson Cancer Center
Toronto University
Toronto University
UCSF (University of California, San Francisco)
United Therapeutics
University of California, Berkeley
University of Colorado Cord Blood Bank
University of Tokyo
US Center for Disease Prevention and Control (CDC)
US Food and Drug Administration
Walter and Eliza Hall Institute
World Bank
World Health Organization (WHO)
Yonsei University
Download sample pages
Complete the form below to download your free sample pages for Biologics Market Trends and Forecasts 2018-2028
Related reports
-

Global Bariatric Surgery Devices Market Forecast 2018-2028
The Global Bariatric Surgery Devices Market is estimated at $879.7m in 2017 and is expected to grow at a CAGR...
Full DetailsPublished: 08 February 2018 -

Top 20 Vaccines Manufacturers 2019
The global vaccines market has witnessed strong growth in past few years. The top 5 manufacturers in the global vaccines...
Full DetailsPublished: 12 February 2019 -

Drug Delivery Technologies Market Forecast 2019-2029
The Drug Delivery Technologies market is estimated to grow at a CAGR of 8.3% in the first half of the...
Full DetailsPublished: 27 February 2019 -

Global Vaccine Contract Manufacturing Market Report 2018-2028
The global vaccine contract manufacturing market was worth $883.0m in 2017 and is expected to grow at a CAGR of...
Full DetailsPublished: 06 March 2018 -

Global Bioreactors Market 2019-2029
The global bioreactors market is expected to grow at a CAGR of 7.5% in the second half of the forecast...
Full DetailsPublished: 18 December 2018 -

Generic Drugs Market Forecast 2018-2028
The generic drugs market is estimated at $257.3bn in 2017 and is expected to grow at a CAGR of 7.9%...
Full DetailsPublished: 25 April 2018 -

Pharma Leader Series: 25 Top Biosimilar Drug Manufacturers 2017-2027
Our 233-page report provides 126 tables, charts, and graphs, giving a clear view on companies in the biosimilar market. Who...Full DetailsPublished: 31 August 2017 -

Global Translational Regenerative Medicine Market Prospects 2018-2028
The Global Translational Regenerative Medicine market is estimated to grow at a CAGR of 22.1% in the first half of...
Full DetailsPublished: 27 March 2018 -

Global Influenza Vaccines Market Outlook 2018-2028
The latest report from business intelligence provider visiongain offers comprehensive analysis of the global influenza vaccines market. Visiongain assesses that...
Full DetailsPublished: 19 June 2018 -

Global Diabetes Drugs Market 2017-2027
The global diabetes drugs market was valued at $49.26bn in 2016 and is projected to grow at a CAGR of...Full DetailsPublished: 27 July 2017
Download sample pages
Complete the form below to download your free sample pages for Biologics Market Trends and Forecasts 2018-2028
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Don’t Miss Out!
Subscribe to receive the latest Industry news, trending insight and analysis straight to your inbox.Choose your preferences:
Latest Pharma news
Retinal Gene Therapy Market
The global Retinal Gene Therapy market is projected to grow at a CAGR of 9.6% by 2034
26 July 2024
HIV Drugs and Injectables Market
The global HIV Drugs & Injectables market is projected to grow at a CAGR of 4.6 % by 2034
24 July 2024
Digital Twin Technology in Pharmaceutical Manufacturing Market
The global Digital Twin Technology in Pharmaceutical Manufacturing market is projected to grow at a CAGR of 31.3% by 2034
23 July 2024
Specialty Pharma Market
The global Specialty Pharma market is projected to grow at a CAGR of 7.5% by 2034
22 July 2024

















