Industries > Biosimilars Market Size Reports > Global Next-Generation Antibody Therapies Market Forecast 2018-2028
Global Next-Generation Antibody Therapies Market Forecast 2018-2028
Antibody-Drug Conjugates, Engineered Antibodies, Bispecific Antibodies, Antibody Fragments & ALPs, Biosimilar Antibodies
The global next-generation antibody therapies market reached $4bn in 2017 and is estimated to reach $17bn by 2023. In 2017, the antibody-drug conjugates segment held 45% of the global next-generation antibody therapies market.
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in this sector.
In this brand new 217-page report you will receive 141 charts– all unavailable elsewhere.
The 217-page report provides clear detailed insight into the next-generation antibody therapies market. Discover the key drivers and challenges affecting the market.
By ordering and reading our brand-new report today you stay better informed and ready to act.
Report Scope
• Global Next-Generation Antibody Therapies Market from 2018-2028
• Forecast of the Global Next-Generation Antibody Therapies market by Submarket:
• Antibody Fragments & ALPs
• Antibody-Drug Conjugates
• Biosimilar Antibodies
• Bispecific Antibodies
• Engineered Antibodies
• Forecast of the Global Antibody Fragments & ALPs submarket by Product:
• Kalbitor
• Other Antibody Fragments
• Forecast of the Global Antibody-Drug Conjugates submarket by Product:
• Adcetris
• Kadcyla
• Mylotarg
• Other ADCs
• Forecast of the Global Biosimilar Antibodies submarket by Product:
• Infliximab
• Adalimumab
• Rituximab
• Bevacizumab
• Trastuzumab
• Other Biosimilar mAbs
• Forecast of the Global Bispecific Antibodies submarket by Product:
• Blincyto
• Hemlibra
• Other Bispecific Antibodies
• Forecast of the Global Engineered Antibodies submarket by Product:
• Gazyva/Gazyvaro
• Poteligeo
• Fasenra
• Other Engineered Antibodies
• This report provides individual revenue forecasts to 2028 for these regional and national markets:
• North America: US, Canada, and Mexico
• Europe: Germany, France, United Kingdom, Italy, Spain, Russia, and rest of Europe
• Asia-Pacific: Japan, China, Australia, South Korea, India, and rest of Asia-Pacific
• LAMEA: Brazil, Saudi Arabia, Republic of South Africa, Turkey, and rest of LAMEA
• Our study discusses the selected leading companies that are the major players in the next-generation antibody therapies market:
• Amgen, Inc.
• GlaxoSmithKline (GSK)
• Kirin Holdings (Kyowa Hakko Kirin Co., Ltd.)
• Merck KGaA
• Novartis AG
• Pfizer, Inc.
• Roche
• Sanofi
• Seattle Genetics, Inc.
• Shire plc
• This report discusses SWOT & STEP analysis of the next-generation antibody therapies market as well as factors that drive and restrain this market. This report also discusses the opportunities that can be tapped in this market.
• This report also discusses selected at that are in the pipeline.
Visiongain’s study is intended for anyone requiring commercial analyses for the next-generation antibody therapies market. You find data, trends and predictions.
Buy our report today Global Next-Generation Antibody Therapies Market Forecast 2018-2028: Antibody-Drug Conjugates, Engineered Antibodies, Bispecific Antibodies, Antibody Fragments & ALPs, Biosimilar Antibodies
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
1. Report Overview
1.1 Global Next-Generation Antibody Therapies Market Overview
1.2 Global Next-Generation Antibody Therapies Market Segmentation
1.3 Why you Should Read this Report?
1.4 How this Report Delivers
1.5 Main Questions Answered by This Analytical Study
1.6 Who is This Report For?
1.7 Methodology
1.8 Frequently Asked Questions (FAQ)
1.9 Associated Reports
1.10 About Visiongain
2. Introduction to Next-Generation Antibody Therapies
2.1 Market Definition & Scope of this Report
2.2 Antibodies: An Overview
2.2.1 Monoclonal vs Polyclonal Antibodies
2.2.2 A Brief History of Antibody Drug Development
2.2.3 The Antibody Manufacturing Process
2.2.4 Trends in Antibody Development
2.3 Defining Next-Generation Antibodies
2.4 Classification of Next-Generation Antibody Therapies
2.5 The Need for Next-Generation Technologies
2.6 Development Trends for Next-Generation Antibodies
2.7 Phases of Clinical Trials
3. Global Next-Generation Antibody Therapies Market, 2017-2028
3.1 The Global Next-Generation Antibody Therapies Market Overview and Segmentation, 2017
3.2 Leading Next-Generation Antibody Therapies
3.3 Global Next-Generation Antibody Therapies Market: Sales Forecast 2017-2028
3.4 How Will Segments Market Shares Change to 2028?
3.5 Global Next-Generation Antibody Therapies Market: Drivers and Restraints 2017-2028
3.6 Global Next-Generation Antibody Therapies Market by Product Type, 2017-2028
4. Global Next-Generation Antibody Therapies Market by Product Type: Revenue Forecast, 2018-2028
4.1 Antibody-Drug Conjugates Submarket
4.1.1 Global Antibody-Drug Conjugates Submarket: Revenue Forecast, 2018-2028
4.2 Engineered Antibodies Submarket
4.2.1 Global Engineered Antibodies Submarket: Revenue Forecast, 2018-2028
4.3 Bispecific Antibodies Submarket
4.3.1 Global Bispecific Antibodies Submarket: Revenue Forecast, 2018-2028
4.4 Antibody Fragments and ALPs
4.4.1 Global Antibody Fragments and ALPs Revenue Submarket: Forecast, 2018-2028
4.5 Biosimilar Antibody Submarket
4.5.1 Global Biosimilar Antibody Submarket: Revenue Forecast, 2018-2028
4.6 Segment Share Analysis by Product Type
5. Global Next-Generation Antibody Therapies Market by Regional and National Market: Revenue Forecast, 2018-2028
5.1 Global Next-Generation Antibody Therapies Market by Region: Revenue Forecast, 2018-2028
5.2 North America
5.2.1 North America Next-Generation Antibody Therapies Market by Country: Revenue Forecast, 2018-2028
5.2.2 US Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.2.3 Canada Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.2.4 Mexico Next-Generation Antibody Therapies Market, Revenue Forecast, 2018-2028
5.3 Europe
5.3.1 Europe Next-Generation Antibody Therapies Market by Country: Revenue Forecast, 2018-2028
5.3.2 Germany Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.3.3 France Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.3.4 United Kingdom Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
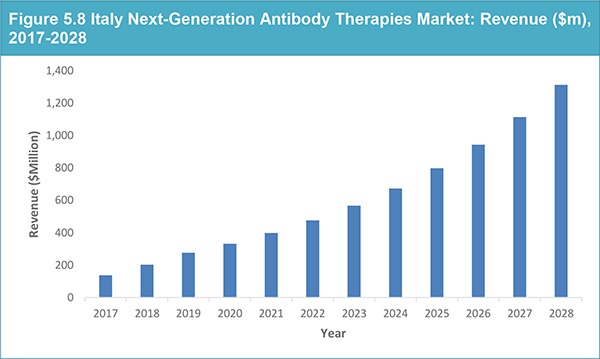
5.3.5 Italy Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.3.6 Spain Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.3.7 Russia Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.4 Asia-Pacific
5.4.1 Asia-Pacific Next-Generation Antibody Therapies Market by Country: Revenue Forecast, 2018-2028
5.4.2 Japan Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.4.3 China Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.4.4 Australia Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.4.5 South Korea Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.4.6 India Next-Generation Antibody Therapies Market, Revenue Forecast, 2018-2028
5.5 LAMEA
5.5.1 LAMEA Next-Generation Antibody Therapies Market by Country: Revenue Forecast, 2018-2028
5.5.2 Brazil Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.5.3 Saudi Arabia Next-Generation Antibody Therapies Market: Revenue Forecast, 2018-2028
5.5.4 Republic of South Africa Next-Generation Antibody Therapies Market, Revenue Forecast, 2018-2028
6. Antibody-Drug Conjugates: Market Forecast and Pipeline 2017-2028
6.1 Market Overview
6.2 Leading ADCs in 2017
6.3 Antibody-Drug Conjugates: Market Forecast 2017-2028
6.4 Adcetris
6.4.1 Adcetris: Sales 2013-2017
6.4.2 Adcetris Results in AETHERA Trial and Approval for in HL following Stem Cell Transplantation
6.4.3 Expanding Adcetris Indications for Future Revenue Growth
6.4.4 Adcetris Sales Forecast 2018-2028
6.5 Kadcyla
6.5.1 Mixed Results from Different Clinical Trials
6.5.2 NICE Rejects Kadcyla on Cost Grounds, but it Just Manages to Hold on in the Cancer Drugs Fund List
6.5.3 Kadcyla Sales Forecast 2018-2028
6.6 Mylotarg
6.6.1 Mylotarg Sales Forecast 2018-2028
6.7 Antibody-Drug Conjugate Platforms
6.7.1 Radioconjugation
6.7.2 Seattle Genetics’ Platform
6.7.3 ImmunoGen: TAP Technology
6.7.4 Immunomedics
6.7.5 Preclinical Platforms for ADC Development
6.8 ADCs Have Attracted High-Value Deals and Will Continue to do so
6.9 Antibody-Drug Conjugates: Pipeline Analysis 2017-2028
6.9.1 The Longest Pipeline in the Next-Generation Antibodies Market
6.9.2 Cancer Is the Only Target for Current Clinical-Stage ADCs
6.9.3 Seattle Genetics’ Phase 1 and Phase 2 ADCs
6.9.3.1 SGN-LIV1A (anti-LIV-1) for Relapsed Breast Cancer
6.10 Antibody-Drug Conjugates: Phase 3 Pipeline
6.10.1 CMC544 (inotuzumab ozogamicin, Pfizer)
6.10.1.1 Granted Breakthrough Therapy Designation
6.10.2 Roche Attempting to Expand Kadcyla Indications and also Possesses some Unique ADCs in the Pipeline
6.10.2.1 RG7596 (polatuzumab vedotin) for NHL and DLBCL
6.11 Antibody-Drug Conjugates: Phase 2 Pipeline
6.11.1 ABT-414 (anti-EGFR, AbbVie/Seattle Genetics)
6.11.2 BT-062 (indatuximab ravtansine; anti-CD138, Biotest)
6.11.3 Immunomedics’ IMMU-130 (labetuzumab-SN-38; anti-CEA/CD66e) and IMMU-132 (anti-TROP-2)
6.11.4 PSMA ADC (anti-PSMA, Progenics Pharmaceuticals/ Seattle Genetics)
6.12 Antibody-Drug Conjugates: Phase 1 Pipeline
6.13 Antibody-Drug Conjugates: Preclinical Pipeline
6.14 Future Developments in ADC Technology
6.14.1 Site-Specific Linkage for Improved Safety Profiles
7. Engineered Antibodies: Market Forecast 2017-2028
7.1 Market Overview
7.2 Leading Engineered Antibodies in 2017
7.3 Engineered Antibodies: Market Forecast 2017-2028
7.4 Gazyva/Gazyvaro
7.4.1 Gazyva/Gazyvaro Sales Forecast, 2018-2028
7.5 Poteligeo
7.5.1 Poteligeo Sales Forecast, 2018-2028
7.6 Engineered Antibodies Platforms
7.6.1 Roche Glycart: GlucoMAb
7.6.2 Kyowa Hakko Kirin: Potelligent
7.6.3 Glycotope: GlycoExpress
7.6.4 MacroGenics
7.6.5 Xencor: XmAb
7.7 Engineered Antibodies: Phase 3 Pipeline
7.7.1 Ublituximab (anti-CD20, TG Therapeutics)
7.7.2 MEDI-551 (anti-CD19, AstraZeneca)
7.7.3 MOR-208 (XmAb5574) (anti-CD19, MorphoSys/Xencor)
7.8 Engineered Antibodies: Phase 2 Pipeline
7.8.1 Glycotope’s CetuGEX (anti-EGFR), PankoMab-GEX (anti-TA-MUC1) and TrasGEX (anti-HER2)
7.8.2 Margetuximab (anti-HER2, MacroGenics)
7.8.3 Teplizumab (anti-CD3, MacroGenics)
7.8.4 XmAb5871 (anti-CD19, Xencor)
7.9 Engineered Antibodies: Phase 1 Pipeline
8. Bispecific Antibodies: Market Forecast 2017-2028
8.1 Market Overview
8.2 Bispecific Antibodies: Market Forecast 2017-2028
8.3 Blincyto
8.3.1 Blincyto Sales Forecast, 2018-2028
8.4 Bispecific Antibodies Platforms
8.4.1 BiTE Platform
8.4.2 MacroGenics’ DART Platform
8.4.3 TriomAbs (TRION Pharma)
8.4.4 Other Bispecific Antibody Platforms
8.4.4.1 DuoBodies (Genmab)
8.4.4.2 ImmTAC (Immunocore)
8.5 Bispecific Antibodies: Pipeline Analysis 2017-2028
8.6 Bispecific Antibodies: Phase 2 and Phase 3 Pipeline
8.6.1 ABT-981 (anti-IL-1α and IL-1β, AbbVie)
8.6.2 AFM13 (anti-CD30 and CD16A, Affimed Therapeutics)
8.6.3 MM-141 (anti-IGF-1R and ErbB3, Merrimack Pharmaceuticals)
8.6.4 SAR156597 (anti-IL-4 and IL-13, Sanofi)
8.7 Bispecific Antibodies: Phase 1, Phase 1/2 and Preclinical Pipeline
9. Antibody Fragments and Antibody-Like Proteins (ALPs): Market Forecast 2017-2028
9.1 Market Overview
9.2 Antibody Fragments and Antibody-Like Proteins (ALPs): Market Forecast 2017-2028
9.3 Kalbitor
9.3.1 Kalbitor Sales Forecast, 2018-2028
9.4 Antibody Fragments and ALPs Platforms
9.4.1 Single-chain Variable Fragment Platforms
9.4.1.1 ESBATech and Delenex Therapeutics
9.4.1.2 Nanobodies: The Smallest Antibody Fragment
9.4.1.3 Ablynx’s Nanobody Platform
9.4.1.4 Domain Antibodies: GSK and Crescendo Biologics
9.4.2 Non-Antibody Protein Scaffolds: Antibody-Like Proteins
9.4.2.1 DARPins: One-Tenth the Size of Antibodies
9.4.2.2 Anticalins (Pieris)
9.4.2.3 Affibodies (Affibody)
9.4.2.4 Fynomers (Covagen/Johnson & Johnson)
9.4.2.5 Affilins (Scil Proteins)
9.4.2.6 Adnectins (Bristol-Myers Squibb)
9.4.2.7 AdAlta: i-bodies
9.5 Antibody Fragments and ALPs: Pipeline Analysis 2017-2028
9.5.1 Ablynx Leads the Pipeline with Eight Clinical Projects
9.6 Antibody Fragments and ALPs: Phase 3 Pipeline
9.6.1 Caplacizumab (anti-vWF, Ablynx)
9.6.2 Abicipar (anti-VEGF, Allergan/Molecular Partners)
9.7 ESBA1008 (anti-VEGF, Novartis)
9.8 Antibody Fragments and ALPs: Phase 2 Pipeline
9.8.1 ALX-0061 (anti-IL-6R, Ablynx/AbbVie)
9.8.2 Ozoralizumab (anti-TNFα, Ablynx )
9.8.3 DLX105 (anti-TNF-α, Delenex Therapeutics)
9.8.4 ALX-0171 (anti-respiratory syncytial virus)
9.8.5 PRS-080 (Pieris Pharmaceuticals GmbH)
9.9 Antibody Fragments and ALPs: Phase 1 Pipeline
9.9.1 ALX-0761/M1095 (anti-IL-17A and IL-17F, Ablynx/Merck Serono)
9.9.2 ALX-0141 (anti-RANKL, Ablynx/Eddingpharm)
9.10 Other Phase 1 Candidates, and the Preclinical Pipeline
10. Biosimilar Antibodies: Market Forecast 2017-2028
10.1 Market Overview
10.2 Leading Biosimilar Antibodies in 2017
10.3 Biosimilar Antibodies: Market Forecast 2017-2028
10.4 Infliximab Biosimilar Sales Forecast, 2018-2028
10.5 Adalimumab Biosimilar Sales Forecast, 2018-2028
10.6 Rituximab Biosimilar Sales Forecast, 2018-2028
10.7 Trastuzumab Biosimilar Sales Forecast, 2018-2028
10.8 The Biosimilar Antibodies Market: Drivers and Restraints 2017-2028
10.8.1 New Launches of Biosimilar mAbs in Developed and Emerging Markets
10.8.2 Rising Incidence of Cancer Will Drive Demand
10.8.3 Novel mAb Developers Choosing to Develop Biobetters and Next-Generation Therapies in Face of Biosimilar Competition
10.8.4 Will Biosimilars Challenge Next-Generation Antibodies in this Decade?
11. Leading Companies in Next-Generation Antibody Therapies Market
11.1 Seattle Genetics Inc.
11.1.1 Company Overview
11.1.2 Operating Segments
11.1.3 Financial Highlights
11.1.4 Product Pipeline
11.1.5 Recent Strategic Developments
11.2 F. Hoffmann-La Roche Ltd.
11.2.1 Company Overview
11.2.2 Operating Segments
11.2.3 Financial Highlights
11.2.4 Product Pipeline
11.2.5 Recent Strategic Developments
11.3 Pfizer, Inc.
11.3.1 Company Overview
11.3.2 Operating Segments
11.3.3 Financial Highlights
11.3.4 Recent Strategic Developments
11.4 Kirin Holdings (Kyowa Hakko Kirin Co., Ltd.)
11.4.1 Company Overview
11.4.2 Operating Segments
11.4.3 Financial Highlights
11.4.4 Recent Strategic Developments
11.5 Sanofi S.A.
11.5.1 Company Overview
11.5.2 Operating Segments
11.5.3 Financial Highlights
11.5.4 Recent Strategic Developments
11.6 Merck KGaA (Merck Sereno)
11.6.1 Company Overview
11.6.2 Operating Segments
11.6.3 Financial Highlights
11.6.4 Recent Strategic Developments
11.7 Amgen Inc.
11.7.1 Company Overview
11.7.2 Operating Segments
11.7.3 Financial Highlights
11.7.4 Recent Strategic Developments
11.8 Novartis AG
11.8.1 Company Overview
11.8.2 Operating Segments
11.8.3 Financial Highlights
11.8.4 Recent Strategic Developments
11.9 GlaxoSmithKline plc
11.9.1 Company Overview
11.9.2 Operating Segments
11.9.3 Financial Highlights
11.10 Shire plc
11.10.1 Company Overview
11.10.2 Operating Segments
11.10.3 Financial Highlights
12. Qualitative Analysis of the Next-Generation Antibody Therapies Market 2017-2028
12.1 SWOT Analysis of the Next-Generation Antibody Therapies Market
12.1.1 Strengths: The Path Towards Market Acceptance
12.1.2 Weaknesses – Multiple Companies Targeting Similar Indications
12.1.2.1 Challenges Exist with Current Monoclonal Antibody Therapies
12.1.3 Opportunities: Novel Platforms
12.1.3.1 Big Pharmas are Investing Heavily in Next-Generation Pipelines
12.1.4 Threats: Entry of Biosimilars
12.1.4.1 Will Biosimilars Growth Slow Down in the Next-Generation Antibody Market?
12.2 Market Dynamics
12.2.1 Drivers
12.2.1.1 Rise in prevalence of cancer diseases
12.2.1.2 Increase in medical insurance coverage
12.2.1.3 Strong pipeline of drugs
12.2.2 Restraints
12.2.2.1 Risk of allergic reaction and limitations
12.2.2.2 Lengthy & stringent government regulations
12.2.3 Opportunities
12.2.3.1 Increasing R&D in cancer therapy
12.2.3.2 Increase in purchasing power of emerging economies
12.3 PEST Analysis of the Next-Generation Antibody Therapies Market
12.3.1 Political and Regulatory Issues
12.3.1.1 Regulatory Challenges for Biosimilar Antibodies
12.3.2 Economic Pressures
12.3.2.1 Next-Generation Antibodies Are High-Cost
12.3.2.2 Outsourced Manufacturing: CMOs Are Expanding Capabilities
12.3.2.3 Next-Generation Launches for Product Lifecycle Management
12.3.3 Social Factors
12.3.3.1 Cancer Incidence Is Rising Rapidly
12.3.3.2 Next-Generation Antibodies for Personalised Medicine
12.3.4 Technological Developments
12.3.4.1 There Are Many Competing Platforms
12.3.4.2 Manufacturing Challenges Exist for Most Sectors
12.3.4.3 New Analytical Tools for Target Identification and Protein Characterisation
12.4 Key Targets for Next-Generation Antibody Development 2017-2028
12.4.1 Oncology Is the Lead Indication in All Sectors
12.4.1.1 HER2 and HER3 for Breast Cancer
12.4.1.2 CD19 and CD20 for Lymphoma and Leukaemia
13. Conclusions
13.1 High Growth Potential in the Next-Generation Antibody Therapies Market in 20167-2028
13.2 Current Status of the Market and Leading Segments
13.3 Leading Next-Generation Antibody Therapies Profiled in this Report
13.4 Leading Regional Markets
13.5 Development of the Market to 2028
13.6 Technology Platforms Will Continue to Attract Big Pharma Interest
13.7 Most Developers Continue to Target Cancer
13.8 Strategies for Growth in 2017-2028
Appendices
Associated Visiongain Reports
Visiongain Report Sales Order Form
About Visiongain
Visiongain report evaluation form
List of Tables
Table 2.1 Monoclonal Antibody Types and Sources
Table 2.2 The Different Phases of Clinical Trials and What They Assess
Table 3.1 Global Next-Generation Antibody Therapies Market by Product: Revenue ($m) and Market Share (%), 2017
Table 3.2 Global Next-Generation Antibody Therapies Market Forecast: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 3.3 Global Next-Generation Antibody Therapies Market by Product: Revenue ($m) and Market Share (%), 2017 & 2028
Table 4.1 Global Next-Generation Antibody Therapies Market by Product Type: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 4.2 Global Antibody-Drug Conjugates Submarket: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 4.3: Global Engineered Antibodies Submarket: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 4.4 Global Bispecific Antibodies Submarket: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 4.5 Global Antibody Fragments and ALPs Submarket: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 4.6 Global Biosimilar Antibody Submarket: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 4.7 Global Next-Generation Antibody Therapies Market by Product Type: Revenue ($m), Revenue Share (%), 2017, 2023, 2028
Table 5.1 Global Next-Generation Antibody Therapies Market by Region: Revenue ($m), Revenue Share (%), 2017, 2023, 2028
Table 5.2 Global Next-Generation Antibody Therapies by Leading National Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.3 North America Next-Generation Antibody Therapies Market by Country: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.4 US Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.5 Canada Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.6 Mexico Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.7 Europe Next-Generation Antibody Therapies Market by Country: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.8 Germany Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.9 France Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.10 United Kingdom Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.11 Italy Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.12 Spain Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.13 Russia Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.14 Asia-Pacific Next-Generation Antibody Therapies Market by Country: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.15 Japan Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.16 China Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.17 Australia Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.18 South Korea Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.19 India Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.20 LAMEA Next-Generation Antibody Therapies Market by Country: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.21 Brazil Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.22 Saudi Arabia Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 5.23 Republic of South Africa Next-Generation Antibody Therapies Market: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 6.1 Antibody-Drug Conjugates Market by Type: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 6.2 Adcetris: Market Revenue ($m) 2013-2017
Table 6.3 Adcetris Market Revenue ($m), AGR (%) and CAGR (%) 2018-2028
Table 6.4 Kadcyla: Market Revenue ($m), AGR (%) and CAGR (%) 2018-2028
Table 6.5 Mylotarg Market Revenue ($m), AGR (%) and CAGR (%) 2018-2028
Table 6.6 Seattle Genetics ADC Collaborator Pipeline
Table 6.7 Selected Preclinical ADC Development Platforms
Table 6.8 ADC Pipeline by Major Indications, 2017
Table 6.9 ADC Phase 3 Pipeline by Indication, 2018
Table 6.10 Selected Ongoing Clinical Trials for CMC544 (inotuzumab ozogamicin), 2018
Table 6.11 Roche’s Antibody-Drug Conjugates Pipeline, 2018
Table 6.12 Selected Ongoing Clinical Trials for RG7596 (polatuzumab vedotin), 2018
Table 6.13 ADC Phase 2 Pipeline by Indication, 2018
Table 6.14 Selected Ongoing Clinical Trials for ABT-414, 2018
Table 6.15 ADC Phase 1 Pipeline by Indication, 2018
Table 6.16 ADC Preclinical Pipeline, 2018
Table 7.1 Engineered Antibodies Market by Type: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 7.2 Gazyva/Gazyvaro Market: Revenue ($m), AGR (%) and CAGR (%) 2018-2028
Table 7.3 Poteligeo Market: Revenue ($m), AGR (%) and CAGR (%) 2018-2028
Table 7.4 Engineered Antibodies Phase 3 Pipeline by Indication, 2018
Table 7.5 Selected Ongoing Clinical Trials for MEDI-551, 2018
Table 7.6 Engineered Antibodies Phase 2 Pipeline by Indication, 2018
Table 7.7 Selected Ongoing Clinical Trials for Margetuximab, 2018
Table 7.8 Engineered Antibodies Phase 1 Pipeline by Indication, 2018
Table 8.1 Bispecific Antibodies Market by Type: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 8.2 Blincyto: Market Revenue ($m), AGR (%) and CAGR (%) 2018-2028
Table 8.3 Bispecific Antibodies Pipeline by Indication and Phase, 2017
Table 8.4 Bispecific Antibodies Pipeline by Indication and Phase, 2018
Table 8.5 Bispecific Antibodies: Phase 1/2 Pipeline, 2018
Table 8.6 Bispecific Antibodies: Phase 1 Pipeline, 2018
Table 8.7 Bispecific Antibodies: Preclinical Pipeline, 2018
Table 9.1 Antibody Fragments and Antibody-Like Proteins (ALPs) Market by Type: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 9.2: Kalbitor Market: Revenue ($m), AGR (%) and CAGR (%) 2018-2028
Table 9.3 Antibody Fragments and ALPs Pipeline, 2018
Table 10.1 Biosimilar Antibody Market by Type: Revenue ($m), AGR (%) and CAGR (%) 2017-2028
Table 10.2 Infliximab Biosimilar Market: Revenue ($m), AGR (%) and CAGR (%) 2018-2028
Table 10.3 Adalimumab Biosimilar Market: Revenue ($m), AGR (%) and CAGR (%) 2018-2028
Table 10.4 Rituximab Biosimilar Market: Revenue ($m), AGR (%) and CAGR (%) 2018-2028
Table 10.5 Trastuzumab Biosimilar Market: Revenue ($m), AGR (%) and CAGR (%) 2018-2028
Table 10.6 Patent Status of Five Leading mAbs
Table 11.1 Seattle Genetics Inc. Operating Segments
Table 11.2 Seattle Genetics Inc.: Revenue ($m), R&D ($m), 2015-2017
Table 11.3 Seattle Genetics Inc. Product Pipeline
Table 11.4 Seattle Genetics Inc. Recent Developments
Table 11.5 Roche Operating Segments
Table 11.6 Roche: Revenue ($m), R&D ($m), 2015-2017
Table 11.7 Roche Product Pipeline
Table 11.8 Roche Recent Developments
Table 11.9 Pfizer Operating Segments
Table 11.10 Pfizer: Revenue ($m), R&D ($m), 2015-2017
Table 11.11 Pfizer Recent Developments
Table 11.12 Kyowa Hakko Kirin Operating Segments
Table 11.13 Kyowa Hakko Kirin: Revenue ($m), R&D ($m), 2015-2017
Table 11.14 Kyowa Hakko Kirin Recent Developments
Table 11.15 Sanofi Operating Segments
Table 11.16 Sanofi: Revenue ($m), R&D ($m), 2015-2017
Table 11.17 Sanofi Recent Developments
Table 11.18 Merck Operating Segments
Table 11.19 Merck: Revenue ($m), R&D ($m), 2015-2017
Table 11.20 Merck Recent Developments
Table 11.21 Amgen Operating Segments
Table 11.22 Amgen: Revenue ($m), R&D ($m), 2015-2017
Table 11.23 Amgen Recent Developments
Table 11.24 Novartis Operating Segments
Table 11.25 Novartis: Revenue ($m), R&D ($m), 2015-2017
Table 11.26 Novartis Recent Developments
Table 11.27 GSK Operating Segments
Table 11.28 GSK: Revenue ($m), R&D ($m), 2015-2017
Table 11.29: Shire Operating Segments
Table 11.30: Shire: Revenue ($m), R&D ($m), 2015-2017
List of Figures
Figure 1.1 Global Next-Generation Antibody Therapies Market, Market Segmentation
Figure 3.1 Global Next-Generation Antibody Therapies by Product: Market Share (%), 2017
Figure 3.2 Global Next-Generation Antibody Therapies Market Forecast Revenue ($m) & AGR (%), 2017-2028
Figure 3.3 Global Next-Generation Antibody Therapies Market by Product: Revenue ($m), 2028
Figure 3.4 World Next-Generation Antibody Therapies Market: Drivers and Restraints, 2017-2028
Figure 3.5 Global Next-Generation Antibody Therapies Market by Product: Revenue ($m), 2017-2028
Figure 4.1 Global Antibody-Drug Conjugates Submarket: Revenue ($m), 2017-2028
Figure 4.2 Global Engineered Antibodies Submarket: Revenue ($m), 2017-2028
Figure 4.3 Global Bispecific Antibodies Submarket: Revenue ($m), 2017-2028
Figure 4.4 Global Antibody Fragments and ALPs Submarket: Revenue ($m), 2017-2028
Figure 4.5: Global Next-Generation Antibody Therapies Market, Biosimilar Antibody Revenue ($m), 2017-2028
Figure 5.1 Global Next-Generation Antibody Therapies Market by Region: Revenue ($m), 2017-2028
Figure 5.2 US Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.3 Canada Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.4 Mexico Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.5 Germany Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.6 France Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.7 United Kingdom Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.8 Italy Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.9 Spain Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.10 Russia Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.11 Japan Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.12 China Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.13 Australia Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.14 South Korea Next-Generation Antibody Therapies Market, Revenue ($m), 2017-2028
Figure 5.15 India Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.16 Brazil Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.17 Saudi Arabia Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 5.18 Republic of South Africa Next-Generation Antibody Therapies Market: Revenue ($m), 2017-2028
Figure 6.1 Antibody-Drug Conjugates Market Forecast: Revenue ($m) & AGR (%), 2017-2028
Figure 7.1 Engineered Antibodies Market Forecast: Revenue ($m) & AGR (%), 2017-2028
Figure 8.1 Bispecific Antibodies Market Forecast: Revenue ($m) & AGR (%), 2017-2028
Figure 9.1 Antibody Fragments and Antibody-Like Proteins (ALPs) Market Forecast: Revenue ($m) & AGR (%), 2017-2028
Figure 10.1 Biosimilar Antibody Market Forecast: Revenue ($m) & AGR (%), 2017-2028
Figure 12.1 Next-Generation Antibody Therapies Market: Strengths and Weaknesses, 2017-2028
Figure 12.2 Next-Generation Antibody Therapies Market: Opportunities and Threats, 2017-2028
AbbVie
AbGenomics
Ablynx
AdAlta
ADC Therapeutics
Adnexus
Affimed Therapeutics
Agensys
Alcon
Alexion
Algeta ASA
Allergan
ALMAC Group
Ambrx
Amgen
arGEN-X
Astellas Pharma
AstraZeneca
Bayer
Biogen Idec
BioNet Ventures
Biotest
BioWa
Boehringer Ingelheim
Bristol-Myers Squibb
Cancer Drugs Fund
CARBOGEN AMCIS
Cascadian Therapeutics, Inc.
Catalent Pharma Solutions
Celgene
Celldex Therapeutics
Cephalon
Cilag GmbH International
Clovis Oncology
Coldstream Laboratories
Concortis Biosystems
Corixa
Covagen
Crescendo Biologics
CSL Behring
CVie Therapeutics
CytomX Therapeutics
Daiichi Sankyo
Defiante Farmaceutica
Delenex Therapeutics
Domantis
Dutalys
Dyax
Eddingpharm
Eisai
Eli Lilly
Emergent BioSolutions
Endo Pharmaceuticals
ESBATech
Esperance Pharmaceuticals
ETH Zurich
FDA
Five Prime Therapeutics
Formation Biologics
Fresenius Biotech
F-Star
Fujifilm
Genentech
Genmab
Gilead Sciences
Glenmark
Glycart
Glycotope
Goodwin Biotechnology
GSK
Health Canada
Igenica
Immune Design
Immunocore
ImmunoGen
Immunomedics
IONTAS
Janssen Biotech
Jazz Pharmaceuticals
Kalobios
Karolinska Institute
Kyowa Hakko Kirin
Kyowa Medex
LINDIS Biotech
Lonza
MacroGenics
MedImmune
Merck
Merrimack Pharmaceuticals
Mersana Therapeutics
Merus
Micromet
Millennium Pharmaceuticals
Molecular Partners
MorphoSys
MultiCell Immunotherapeutics (MCIT)
National Institute of Diabetes and Digestive and Kidney Diseases
Neopharm
Neovii Biotech
Nerviano Medical Sciences
NHS
NICE
NMS Group
Nordic Nanovector ASA
Novartis
Novartis Institutes for BioMedical Research (NIBR)
NovImmune
Oxford BioTherapeutics
Oxis Biotech
Pfizer
Pharmacyclics
Pieris
Piramal Enterprises
Piramal Healthcare
PolyTherics
ProBioGen
Progenics
ProStrakan
Redwood BioScience
Roche
Salix
Sanofi
Scil Proteins
Seattle Genetics
Servier
Shinogi
Shire
Sigma-Tau
Simcere Pharmaceutical Group
Sorrento Therapeutics
Spectrum Pharmaceuticals
Spirogen
Stelis Biopharma
Stemcentrx
Strides Arcolab
Sutro Biopharma
Swedish Orphan Biovitrum (Sobi)
Swiss Federal Institute of Technology
Synthon
Synthon Biopharmaceuticals
Takeda
Talon Therapeutics
Teva
TG Therapeutics
The American Society of Hematology
The University of Texas MD Anderson Cancer Center
TRION Pharma
UCB
WHO
Wyeth
Xencor
Zydus Cadila
Zymeworks
Download sample pages
Complete the form below to download your free sample pages for Global Next-Generation Antibody Therapies Market Forecast 2018-2028
Related reports
-

Top 25 Antibiotic Drugs Manufacturers 2018
Visiongain forecasts the antibiotic drugs market to increase to $ 43,841.7m in 2022. The market is estimated to grow at...
Full DetailsPublished: 12 June 2018 -

Biologics Market Trends and Forecasts 2018-2028
The global biologics market is estimated to reach $250bn in 2023. The market is expected to grow at a CAGR...
Full DetailsPublished: 14 November 2018 -

Global Biosimilars and Follow-On Biologics Market 2018-2028
The global biosimilars and follow-on biologics market is estimated to have reached $7.70bn in 2017 and expected to grow at...
Full DetailsPublished: 01 June 2018 -

Biological Drug API Manufacturing Services World Industry and Market Predictions 2018-2028
The biological drug API manufacturing market is estimated to grow at a CAGR of 9.0% in the first half of...
Full DetailsPublished: 06 July 2018 -

Global Medical Device Contract Manufacturing Market Forecast 2018-2028
The global medical device contract manufacturing market was valued at $70bn in 2017. Visiongain forecasts this market to increase to...
Full DetailsPublished: 17 July 2018 -

Pharma Leader Series: Top 50 Pharmaceutical Contract Manufacturing Organisations (CMOs) Market 2018
Contract manufacturing represents the largest sector of the pharma outsourcing industry. Pharmaceutical companies have sought to take advantage of the...Full DetailsPublished: 28 September 2018 -

Biological Drug API Manufacturing Services World Industry and Market Forecast to 2029
The biological drug API manufacturing market is estimated to grow at a CAGR of 9.5% in the first half of...Full DetailsPublished: 07 November 2019 -

Global Melanoma Drugs Market 2018-2028
Our new 149-page report provides 161 tables, charts, and graphs. Discover the most lucrative areas in the industry and the...Full DetailsPublished: 17 April 2018 -

Antibody Drug Conjugates Market Report 2021-2031
Antibody drug conjugate (ADC) technology continues to evolve as a widely successful drug targeting technology for the treatment of cancer....
Full DetailsPublished: 03 November 2020 -

Rheumatoid Arthritis Pricing, Reimbursement & Market Access 2018
This report provides analysis and evaluation of the current and potential economic burden of Rheumatoid Arthritis (RA) in the major...
Full DetailsPublished: 29 March 2018
Download sample pages
Complete the form below to download your free sample pages for Global Next-Generation Antibody Therapies Market Forecast 2018-2028
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: sara.peerun@visiongain.com
If so, please drop an email to Sara Peerun stating your chosen report title to sara.peerun@visiongain.com
What are the dynamic growth sectors? where are the regional business opportunities?
Which technologies will prevail and who are the leading companies succeeding in these sectors and why?
If you want definitive answers to business critical questions, discover Visiongain’s full range of business intelligence reports.
If so, please email Sara Peerun on sara.peerun@visiongain.com or call her today on +44 (0) 20 7549 9987
- Advanced Truck Technologies Market 2019-2029
- Biometric Vehicle Access Technologies Market 2019-2029
- Automotive Composites 2019-2029
- Automotive Vehicle to Everything (V2X) Technologies Market 2019-2029
- Automotive Driver Monitoring Systems (DMS) Market 2019-2029
- Wireless Electric Vehicle Charging (WEVC) Technologies Market 2019-2029
Association of Russian Automakers
Audio Video Bridging (AVB)
China Association Of Automoblie Manufacturers
European Association of Automotive Suppliers
European Automobile Manufacturers’ Association
European Council for Automotive Research and Development
Former Society of Automotive Engineers
German Association of the Automotive Industry
Institute of the Motor Industry
International Organization of Motor Vehicle Manufacturers
In-Vehicle Infotainment (IVI)
Italian Association of the Automotive Industry
Japan Automobile Manufacturers Association
One-Pair Ether-Net
Society of Indian Automobile Manufacturers (SIAM)
Society of Motor Manufacturers and Traders
The International Council For Clean Transport
US National Highway Traffic Safety Administration