Visiongain forecasts that the biosimilar drugs market will grow with a CAGR of 40% from 2018 to 2028. Growth is expected to be rapid during the first-half of the forecasted period due to the patent cliff from years 2012-2019, which will see the patent expiry of many of the most popular biologics in the world. The opening of the US market due to clearer defined guideline regarding biosimilars will lead to greater penetration of biosimilars in this lucrative market, which will help the rapid growth and development of the market.
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in this sector.
In this brand new 220-page report you will receive 117 charts– all unavailable elsewhere.
The 220-page report provides clear detailed insight into the top 25 biosimilar drugs manufacturers. Discover the key drivers and challenges affecting the market.
Report Scope
• Discussion and overview of the leading biologic and biosimilar products, covering:
• Humira
• Remicade
• Enbrel
• Aranesp
• Rituxan
• Adalimumab biosimilars
• Infliximab biosimilars
• Etanercept biosimilars
• Darbepoetin Alpha biosimilars
• Rituximab biosimilars
• Profiles of leading biosimilar companies based in the US, Western Europe and Israel:
• Hospira
• Mylan
• Sandoz
• STADA Arzneimittel
• Teva Pharmaceutical Industries
• Other companies in these regions
• Profiles of leading biosimilar companies based in China:
• 3SBio
• Beijing ShuangLu Pharmaceuticals
• Qilu Pharmaceutical
• Shanghai Fosun Pharmaceuticals
• Tonghua Dongbao
• Other companies in China
• Profiles of leading biosimilar companies based in India:
• Biocon
• Dr. Reddy’s Laboratories
• Intas Biopharmaceuticals.
• Ranbaxy
• Reliance Life Sciences
• Wockhardt
• Zydus Cadila
• Other companies in China
• Profiles of leading biosimilar companies based in Latin America:
• Probiomed
• Biosidus
• Amega Biotech
• Other companies in Latin America
• Profiles of leading biosimilar companies based in the rest of the world:
• Celltrion
• LG Life Sciences
• Dong-A
• Bioton
• Biocad
• Other companies in the rest of the world
• The report provides information and discussion on:
• Company overview & analysis
• Biosimilars Products
• Future Outlook
• Technologies and activities
• R&D Pipelines
• Qualitative analysis: a SWOT and STEP analysis of the biosimilars market. Discussion on factors that drive and restrain the biosimilars market.
• Key questions answered by this report:
• What are its drivers and restraints of the biosimilar drugs market?
• What are the leading biosimilar products in the market and which companies manufacture them?
• Who are the leading biosimilars companies?
• What are their products, developmental candidates and therapeutic applications?
• What is the status of the clinical trials they are undergoing?
• What are the latest news and developments from those companies?
• What other biopharmaceutical companies seem promising within the regions we analyse, having potential to succeed in biosimilar drug development, production and marketing?
• What are leading companies’ biosimilar products and what candidates are in their R&D pipelines?
• What are the social, technological, economic and political forces affecting the world biosimilars market?
Visiongain’s study is intended for anyone requiring commercial analyses for the Top 25 Biosimilar Drugs Manufacturers 2019. You find data, trends and predictions.
Buy our report today Biosimilar Drug Manufacturers: Humira, Remicade, Enbrel, Aranesp, Rituxan, Sandoz, Teva, Hospira, Pfizer, STADA Arzneimittel, Mylan, 3SBio, Qilu Pharmaceutical, Shanghai Fosun Pharmaceutical, Biocon, Dr. Reddy’s Laboratories, Probiomed, Biosidus, Celltrion, LG Life Sciences, Other Companies.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
1. Report Overview
1.1 Overview of the Top Biosimilar Drug Manufacturers
1.2 Why You Should Read This Report
1.3 How This Report Delivers
1.4 Main Questions Answered by This Analytical Study
1.5 Who is This Report For?
1.6 Methodology
1.7 Frequently Asked Questions (FAQ)
1.8 Associated Visiongain Reports
1.9 About Visiongain
2. Biosimilar Drugs: World Market Forecast to 2028
2.1 Introduction to Biologics and Biosimilars
2.1.1 Biologic Drugs Definition and Overview
2.1.2 Biosimilars Drugs Definition and Overview
2.1.3 How do Biosimilars Differ from Generics?
2.2 The World Biosimilar Market, 2018-2028
2.3 Overall World Biosimilars Revenue Forecast, 2018-2028
2.4 Biosimilars – Realising their Potential, Market Drivers
2.4.1 Biosimilar Approval Pathways and Regulations
2.4.2 India Releases New Biosimilar Development Guidelines
2.4.3 Russia: Lack of Regulatory Framework Works Well in the Short Term
2.4.4 ‘Pharma 2020’ Initiative
2.4.5 South Korean Market has benefitted from Early Biosimilar Guidelines
2.4.6 Chinese FDA Publishes Finalised Biosimilar Guidelines in 2016
2.4.7 Brazil: ANVISA’s Biosimilar Regulations Are Similar the EMA’s
2.4.8 The Outlook for Biosimilars in the EU
2.4.9 The Outlook for Biosimilars in Japan
2.4.10 Emerging Markets for Biosimilars
2.4.11 Start-up Pressure on Market Leaders
2.4.12 The Patent Cliff for Biologics
2.5 Biosimilars: Market Restraints
2.5.1 Market Fragmentation for Biosimilar Products
2.5.2 Innovative Biologics – Rendering Biosimilars Useless?
2.5.3 Biobetters – A Growing Threat
2.5.4 BREXIT and Trump Election – A Rising Political Threat
3. Leading Biologic & Biosimilar Products: Overview, 2018
3.1 Humira: Product Overview
3.1.1 Adalimumab Biosimilars – Zydus Cadila
3.1.2 Adalimumab Biosimilars – Amgen-Actavis
3.1.3 Adalimumab Biosimilars – Sandoz
3.1.4 Adalimumab Biosimilars – Momenta Pharmaceuticals
3.1.5 Adalimumab Biosimilars – Pfizer
3.1.6 Adalimumab Biosimilars – Boehringer Ingelheim
3.1.7 Adalimumab Biosimilars – Samsung Biologics and Biogen Inc.
3.2 Remicade: Product Overview
3.2.1 Infliximab Biosimilar – Epirus Pharmaceuticals & Ranbaxy
3.2.2 Infliximab Biosimilar – Celltrion & Hospira & Alvogen
3.3 Enbrel: Product Overview
3.3.1 Etanercept Biosimilar – Cipla & Shanghai CP Guojian
3.3.2 Etanercept Biosimilar – Daiichi Sankyo & Coherus Biosciences
3.4 Aranesp: Product Overview
3.4.1 Darbepoetin Alpha Biosimilar – Dr. Reddy’s Laboratories
3.4.2 Darbepoetin Alpha Biosimilar – Cipla & Hetero Drugs Limited
3.4.3 Darbepoetin Alpha Biosimilar – Hospira
3.5 Rituxan: Product Overview
3.5.1 Rituximab Biosimilar – Sandoz
3.5.2 Rituximab Biosimilar – Amgen
3.5.3 Rituximab Biosimilar – Boehringer Ingelheim
3.5.4 Rituximab Biosimilar – Dr. Reddy’s Laboratories
3.5.5 Rituximab Biosimilar – Pfizer
3.5.6 Rituximab Biosimilar – Difficulties Seen in Trials
4. Leading Biosimilar Manufacturers in the United States, Western Europe and Israel
4.1 Sandoz – Company Overview & Analysis
4.1.1 Sandoz Biosimilar Products
4.1.2 Sandoz Biosimilars Market Forecast, 2018-2028
4.1.3 Sandoz Biosimilars R&D Pipeline & Future Outlook
4.1.3.1 GP2018 – Adalimumab Biosimilar
4.1.3.2 GP2015 – Etanercept Biosimilar
4.1.3.3 GP2013 – Rituximab Biosimilar
4.1.3.4 HX575 – Epoetin-alfa Biosimilar
4.1.3.5 EP2006 – Zarzio (Filgrastim Biosimilar)
4.2 Teva Pharmaceuticals – Company Overview & Analysis
4.2.1 Teva Biosimilar Products
4.2.2 Teva Biosimilar Market Forecast, 2018-2028
4.2.3 Teva Biosimilar Pipeline & Future Outlook
4.3 Hospira (Pfizer) – Company Overview & Analysis
4.3.1 Hospira Biosimilar Products
4.3.2 Hospira Biosimilars Pipeline & Future Strategies
4.3.2.1 Retacrit
4.3.2.2 Celltrion Partnerships
4.3.2.3 NovaQuest Partnership
4.4 STADA Arzneimittel – Company Overview & Analysis
4.4.1 STADA Biosimilar Products
4.4.2 STADA Biosimilars Pipeline & Future Outlook
4.4.2.1 Biosimilar Pegfilgrastim
4.4.2.2 Biosimilar Rituximab
4.4.2.3 Biosimilar Teriparatide
4.5 Mylan – Company Biosimilar & Analysis
4.5.1 Mylan Biosimilar Products
4.5.2 Mylan Biosimilar Market Forecast, 2018-2028
4.5.3 Mylan Biosimilars Pipeline & Future Outlook
4.5.3.1 Mylan and Momenta - Biosimilars Product Development Programme
4.5.3.2 Biosimilar Insulin Glargine
4.5.3.3 A Series of Biosimilar Monoclonal Antibodies
4.5.3.4 Biosimilar Insulin Lispro & Aspart
4.6 Other Biosimilars Companies in the US
4.6.1 Impax Laboratories Inc. – Company Overview & Analysis
4.6.2 Bristol-Myers Squibb – Company Overview & Analysis
4.6.3 Merck & Co. – Company Overview & Analysis
4.6.4 Eli Lilly - Company Overview & Analysis
4.7 Other Biosimilar Companies in Europe
4.7.1 BioXpress Therapeutics – Company Overview & Analysis
4.7.2 Medice Arzneimittel Pütter GmbH & Co. KG – Company Overview & Analysis
4.7.3 Finox Biotech – Company Overview & Analysis
5. Leading Biosimilar Manufacturers in China
5.1 3SBio – Company Overview & Analysis
5.1.1 3SBio Biosimilar Products
5.1.2 3SBio Biosimilars Pipeline & Future Outlook
5.1.2.1 Second-generation rhEPO products
5.1.2.2 Nadroparin Calcium
5.2 Qilu Pharmaceutical – Company Overview & Analysis
5.2.1 Qilu Pharmaceutical Biosimilar Products
5.2.2 Qilu Pharmaceutical Biosimilars Pipeline & Future Outlook
5.2.2.1 Extensive Pipeline Developments
5.2.2.2 Etanercept Biosimilar
5.2.2.3 Other Biosimilars in Pipeline
5.3 Shanghai Fosun Pharmaceuticals – Company Overview & Analysis
5.3.1 Shanghai Fosun Pharmaceuticals Biosimilar Products
5.3.2 Shanghai Fosun Pharmaceuticals Biosimilars Pipeline & Future Strategy
5.3.2.1 Trastuzumab Biosimilar
5.3.2.2 Rituximab Biosimilar
5.3.2.3 Acquisitions and Investments
5.4 Tonghua Dongbao – Company Overview & Analysis
5.4.1 Tonghua Dongbao Biosimilar Products
5.4.2 Tonghua Dongbao Biosimilars Pipeline & Future Strategies
5.4.2.1 Biosimilar Insulin Analogues
5.4.2.2 Investments in New Production Plant
5.5 Beijing ShuangLu Pharmaceuticals – Company Overview & Analysis
5.5.1 Beijing ShuangLu Pharmaceuticals Biosimilar Products
5.5.2 Beijing ShuangLu Pharmaceutical Biosimilars Pipeline & Future Outlook
5.6 Other Biosimilars Companies in China
5.6.1 Shanghai CP Guojian – Company Overview & Analysis
5.6.2 Zhejiang Hisun – Company Overview & Analysis
5.6.3 Innovent Biologics – Company Overview & Analysis
5.6.4 Shanghai Celgen Biopharmaceutical – Company Overview & Analysis
6. Leading Biosimilar Manufacturers in India
6.1 Biocon – Company Overview & Analysis
6.1.1 Biocon Biosimilar Products
6.1.2 Biocon Biosimilars Market Forecast, 2018-2028
6.1.3 Biocon Biosimilars Pipeline & Future Outlook
6.1.3.1 Biosimilar Bevacizumab
6.1.3.2 Biosimilar Adalimumab
6.1.3.3 Biosimilar Pegfilgrastim
6.1.3.4 Biosimilar Trastuzumab
6.1.3.5 Biosimilar Etanercept
6.1.3.6 Biosimilar Recombinant Human Insulin
6.1.3.7 Biosimilar Insulin Glargine
6.1.3.8 Other Biosimilar Insulin
6.2 Dr. Reddy’s Laboratories – Company Overview & Analysis
6.2.1 Dr. Reddy’s Laboratories Biosimilar Products
6.2.2 Dr. Reddy’s Laboratories Biosimilars Pipeline & Future Outlook
6.2.2.1 Merck Serono Collaboration
6.2.2.2 Increasing Focus on Emerging Markets
6.3 Wockhardt – Company Overview & Analysis
6.3.1 Wockhardt Biosimilar Products
6.3.2 Wockhardt Biosimilars Pipeline & Future Outlook
6.4 Zydus Cadila – Company Overview & Analysis
6.4.1 Zydus Cadila Biosimilar Products
6.4.2 Zydus Cadila Biosimilars Pipeline & Future Outlook
6.4.2.1 Biosimilar Interferon beta-1b
6.4.2.2 Oncology Biosimilars
6.4.2.3 Inflammation Biosimilars
6.4.2.4 Thrombolytic Biosimilars
6.4.2.5 Fertility Biosimilars
6.5 Ranbaxy (Sun Pharma) – Company Overview & Analysis
6.5.1 Ranbaxy Biosimilar Products
6.5.2 Ranbaxy Biosimilars Pipeline & Future Outlook
6.6 Reliance Life Sciences – Company Overview & Analysis
6.6.1 Reliance Life Sciences Biosimilar Products
6.6.2 Reliance Life Sciences Biosimilars Pipeline & Future Outlook
6.7 Intas Biopharmaceuticals – Company Overview & Analysis
6.7.1 Intas Biopharmaceuticals Biosimilar Products
6.7.2 Intas Biopharmaceuticals Biosimilars Pipeline & Future Outlook
6.7.2.1 Biosimilar Pegylated Interferon
6.7.2.2 Biosimilar Etanercept
6.7.2.3 Biosimilar Ranibizumab
6.7.2.4 Overseas Opportunities
6.8 Other Indian Biosimilars Companies
6.8.1 Emcure Pharmaceuticals – Company Overview & Analysis
6.8.2 Shreya Life Sciences – Company Overview & Analysis
6.8.3 Cipla – Company Overview & Analysis
6.8.4 Shantha Biotechnics – Company Overview & Analysis
7. Leading Biosimilar Manufacturers in Latin America
7.1 Probiomed – Company Overview & Analysis
7.1.1 Probiomed Biosimilar Products
7.1.2 Probiomed Biosimilars Pipeline & Future Strategies
7.2 Biosidus – Company Overview & Analysis
7.2.1 Biosidus Biosimilar Products
7.2.2 Biosidus Biosimilars Pipeline & Future Strategies
7.3 Amega Biotech – Company Overview and Analysis
7.3.1 Amega – Biosimilar Products
7.3.2 Amega Biotech Biosimilars Pipeline & Future Strategies
7.4 Other Biosimilars Companies in Latin America
7.4.1 Bionovis – Company Overview & Analysis
7.4.2 Orygen Biotecnologia – Company Overview & Analysis
7.4.3 Recepta – Company Overview & Analysis
8. Leading Biosimilar Manufacturers in the Rest of the World
8.1 Celltrion – Company Overview & Analysis
8.1.1 Celltrion Biosimilar Products
8.1.2 Celltrion Biosimilars Pipeline & Future Strategies
8.1.2.1 Trastuzumab Biosimilar
8.1.2.2 Rituximab Biosimilar
8.1.2.3 Four Further Development mAb Biosimilar
8.2 LG Life Sciences – Company Overview & Analysis
8.2.1 LG Life Sciences Biosimilar Products
8.2.2 LG Life Science Biosimilars Pipeline & Future Strategies
8.3 Dong-A – Company Overview & Analysis
8.3.1 Dong-A Biosimilar Products
8.3.2 Dong-A Biosimilars Pipeline & Future Strategies
8.3.2.1 DA-3803 – hCG Biosimilar
8.3.2.2 DA-3031 – Pegylated Filgrastim Biosimilar
8.3.2.3 DA-3880 – Darbepoetin alpha Biosimilar
8.3.2.4 DA-3111 – Trastuzumab Biosimilar
8.3.2.5 DA-3113 – Adalimumab Biosimilar
8.3.2.6 3853 – Etanercept Biosimilar
8.3.2.7 DA-3808 – Recombinant Factor VIII Biosimilar
8.4 – Company Overview & Analysis
8.4.1 Bioton’s Biosimilar Products
8.4.2 Bioton’s Biosimilars Pipeline & Future Strategies
8.5 Biocad – Company Overview & Analysis
8.5.1 Biocad’s Biosimilar Products
8.5.2 Biocad’s Biosimilars Pipeline & Future Strategies
8.6 Other Biosimilars Companies in the RoW
8.6.1 Gedeon-Richter – Company Overview & Analysis
8.6.2 Egis Pharmaceuticals - Company Overview & Analysis
8.6.3 JCR Pharmaceuticals – Company Overview & Analysis
8.6.4 Nippon Kayaku – Company Overview & Analysis
8.6.5 Fujifilm Kyowa Kirin Biologics – Company Overview & Analysis
8.6.6 Daiichi Sankyo – Company Overview & Analysis
9. Qualitative Analysis of Biosimilars Market: 2018-2028
9.1 SWOT Analysis of the Global Biosimilars Industry and Market
9.1.1 Strengths
9.1.1.1 Savings in Healthcare Costs
9.1.1.2 Strong Pipeline – Innovation and Growth of the Biosimilars Market
9.1.1.3 Entry of Biosimilars Into the US market
9.1.2 Weaknesses
9.1.2.1 R&D Cost – Pricing Companies Out of Biosimilars?
9.1.2.2 Developing Market – Biosimilars Still Niche
9.1.2.3 Unclear Regulations for Biosimilars
9.1.3 Opportunities
9.1.3.1 Patent Expiry for Branded Biologics
9.1.3.2 Increase in Prevalence of Chronic Diseases
9.1.3.3 Innovation – New Technology Driving Biosimilar Growth
9.1.3.4 Focus on Biosimilar Monoclonal Antibodies and Insulin
9.1.4 Threats
9.1.4.1 Stricter Regulations for Biosimilar Manufacturers
9.1.4.2 Lawsuits – Patent Protection from Innovator Biologics
9.1.4.3 Biobetters - Removing the Need for Biosimilars?
9.2 STEP Analysis of the Global Biosimilars Industry and Market: 2018 -2027
9.2.1 Social Factors
9.2.1.1 Global Healthcare – An Ambitious Aim?
9.2.1.2 Raising Awareness will Lead to Greater Product Adoption
9.2.2 Technological Factors
9.2.2.1 Innovation Driven – Growing Manufacturing Efficiency
9.2.2.2 High Technological Expertise Needed
9.2.3 Economic Factors
9.2.3.1 R&D for Biosimilar Development – Cost Barriers
9.2.3.2 Healthcare Savings – Cutting Spending and Costs
9.2.4 Political Factors
9.2.4.1 Government Influence – Funding for Biosimilar Industry?
9.2.4.2 International Cooperation Required
9.2.4.3 Regularly Updated Guideline Will Benefit Developers
10. Conclusions
10.1 Market Leaders Among Biosimilar Manufacturers
10.2 Will Sandoz Retain its Position as Market Leader?
10.3 Which Companies Are Best Placed to Lead in Future?
10.4 Big Pharma Companies Will Try Gain Market Entry
10.5 Contribution of Chinese and Indian Companies will Grow
10.6 Strong R&D Pipeline – Driver for Growth
10.7 Concluding Remarks
Appendices
Associated Visiongain Reports
Visiongain Report Sales Order Form
About Visiongain
Visiongain report evaluation form
List of Tables
Table 2.1 Global Biosimilar Market: Revenue Forecast ($bn), Annual Growth (%), CAGR (%), 2018-2028
Table 2.2 Finalised US FDA Biosimilar Guidelines
Table 3.1 Humira Biologic Analysis, 2018
Table 3.2 Remicade Biologic Analysis, 2018
Table 3.3 Enbrel Biologic Analysis, 2018
Table 3.4 Aranesp: Biologic Analysis, 2018
Table 3.5 Rituxan: Biologic Analysis, 2018
Table 4.1 Sandoz: Company Overview, 2018
Table 4.2 Sandoz: Biosimilars Product Portfolio, 2018
Table 4.3 Sandoz: Biosimilar Products Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2028
Table 4.4 Sandoz: Biosimilar Developments Overview, 2018
Table 4.5 Teva: Company Overview, 2018
Table 4.6 Teva: Biosimilars Overview, 2018
Table 4.7 Teva Pharmaceuticals: Biosimilar Products Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2028
Table 4.8 Hospira: Company Overview, 2018
Table 4.9 Hospira: Company Overview, 2018
Table 4.10 Hospira: Biosimilar Developments Overview, 2018
Table 4.13 Stada Arzneimittel: Company Overview, 2018
Table 4.14 Stada Arzneimittel: Biosimilars Overview, 2018
Table 4.15 Stada Arzneimittel: Biosimilar Developments Overview, 2018
Table 4.16 Mylan: Company Overview, 2018
Table 4.17 Mylan: Biosimilars Overview, 2018
Table 4.18 Mylan Pharmaceuticals: Biosimilar Products Forecast: Revenue ($bn), AGR (%), CAGR (%), 2018-2028
Table 4.19 Mylan: Biosimilar Developments Overview, 2018
Table 4.20 Impax Laboratories: Company Overview, 2018
Table 4.21 Bristol-MyersSquibb: Company Overview, 2018
Table 4.22 Merck & Co.: Company Overview, 2018
Table 4.23 Merck & Co.: Biosimilars Product Pipeline, 2018
Table 4.24 Eli Lilly: Company Overview, 2018
Table 4.25 Eli Lilly: Biosimilars Product Portfolio, 2018
Table 4.26 BioXpress Therapeutics: Company Overview, 2018
Table 4.27 Medice Arzneimittel Pütter: Company Overview, 2018
Table 4.28 Finox Biotech: Company Overview, 2018
Table 5.1 3SBio: Company Overview, 2018
Table 5.2 3SBio: Biosimilars Overview, 2018
Table 5.3 3SBio: Biosimilar Developments Overview, 2018
Table 5.4 Qilu Pharmaceutical: Company Overview, 2018
Table 5.5 Qilu Pharmaceutical: Biosimilars Overview, 2018
Table 5.6 Qilu Pharmaceutical: Biosimilar Developments Overview, 2018
Table 5.7 Shanghai Fosun: Company Overview, 2018
Table 5.8 Shanghai Fosun: Biosimilars Overview, 2018
Table 5.9 Shanghai Fosun: Biosimilar Developments Overview, 2018
Table 5.10 Tonghua Dongbao: Company Overview, 2018
Table 5.11 Tonghua Dongbao: Biosimilars Overview, 2018
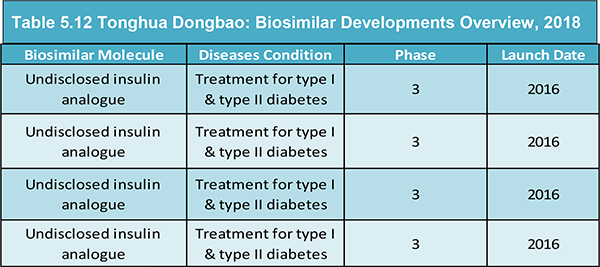
Table 5.12 Tonghua Dongbao: Biosimilar Developments Overview, 2018
Table 5.13 Beijing ShuangLu: Company Overview, 2018
Table 5.14 Beijing ShuangLu: Biosimilars Overview, 2018
Table 5.15 Beijing ShuangLu: Biosimilar Developments Overview, 2018
Table 5.16 Shanghai CP Guojian: Company Overview, 2018
Table 5.17 Zhejiang Hisun: Company Overview, 2018
Table 5.18 Innovent Biologics: Biosimilars Overview, 2018
Table 5.19 Shanghai Celgen Biopharmaceutical: Company Overview, 2018
Table 6.1 Biocon: Company Overview, 2018
Table 6.2 Biocon: Biosimilars Product Portfolio, 2018
Table 6.3 Biocon: Biosimilar Products Forecast: Revenue ($bn), AGR (%), CAGR (%), 2016-2021
Table 6.4 Biocon: Biosimilars Pipeline, 2018
Table 6.5 Dr. Reddy’s Laboratories: Company Overview, 2018
Table 6.6 Dr. Reddy’s Laboratories: Biosimilar Products, 2018
Table 6.7 Dr. Reddy’s Laboratories: Biosimilars Pipeline, 2018
Table 6.8 Wockhardt: Company Overview, 2018
Table 6.9 Wockhardt: Biosimilar Products, 2018
Table 6.10 Wockhardt: Biosimilars Pipeline, 2018
Table 6.11 Zydus Cadila: Company Overview, 2018
Table 6.12 Zydus Cadila: Biosimilars Product Portfolio, 2018
Table 6.13 Zydus Cadila: Biosimilars Pipeline, 2018
Table 6.14 Ranbaxy: Company Overview, 2018
Table 6.15 Ranbaxy: Biosimilars Product Portfolio, 2018
Table 6.16 Zenotech: Biosimilars Pipeline, 2018
Table 6.17 Reliance Life Sciences: Company Overview, 2018
Table 6.18 Reliance Life Sciences: Biosimilar Products, 2018
Table 6.19 Reliance Life Sciences: Biosimilars Pipeline, 2018
Table 6.20 Intas Biopharmaceuticals: Company Overview, 2018
Table 6.21 Intas Biopharmaceuticals: Biosimilar Products Portfolio, 2018
Table 6.22 Intas Biopharmaceuticals: Biosimilars Pipeline, 2018
Table 6.23 Emcure Pharmaceuticals: Company Overview, 2018
Table 6.24 Shreya Life Sciences: Company Overview, 2018
Table 6.25 Cipla: Company Overview, 2018
Table 6.26 Shantha Biotechnics: Company Overview, 2018
Table 7.1 Probiomed: Company Overview, 2018
Table 7.2 Probiomed Biosimilars Product Portfolio, 2018
Table 7.3 Biosidus: Company Overview, 2018
Table 7.4 Biosidus: Biosimilars Product Portfolio, 2018
Table 7.5 Biosidus: Biosimilars Pipeline, 2018
Table 7.6 Amega Biotech: Company Overview, 2018
Table 7.7 Amega Biotech: Biosimilars Product Portfolio , 2018
Table 7.8 Bionovis: Company Overview, 2018
Table 7.9 Orygen Biotechnologia: Company Overview, 2018
Table 7.10 Recepta: Company Overview, 2018
Table 8.1 Celltrion: Company Overview, 2018
Table 8.2 Celltrion: Biosimilars Overview, 2018
Table 8.3 LG Life Sciences: Company Overview, 2018
Table 8.4 LG Life Sciences: Biosimilars Product Portfolio, 2018
Table 8.5 LG Life Sciences: Biosimilars Pipeline, 2018
Table 8.6 Dong-A: Company Overview, 2018
Table 8.7 Dong-A: Biosimilar Product Portfolio, 2018
Table 8.8 Dong-A: Biosimilar Pipeline, 2018
Table 8.9 Bioton: Company Overview, 2018
Table 8.10 Bioton: Biosimilars Product Portfolio, 2018
Table 8.11 Biocad: Company Overview, 2018
Table 8.12 Biocad: Biosimilars Product Portfolio, 2018
Table 8.13 Biocad: Biosimilars Pipeline, 2018
Table 8.14 Gedeon-Richter: Company Overview, 2018
Table 8.15 Egis Pharmaceuticals: Company Overview, 2018
Table 8.16 JCR Pharmaceuticals: Company Overview, 2018
Table 8.17 Nippon Kayaku: Company Overview, 2018
Table 8.18 Fujifilm Kyowa Kirin Biologics: Company Overview, 2018
Table 8.19 Daiichi Sankyo: Company Overview, 2018
List of Figures
Figure 1.1 Biosimilars Market Segmentation, 2018
Figure 2.1 Global Biosimilar Market: Revenue Forecast ($bn), 2018-2028
Figure 2.2 Global Biosimilar Market Drivers, 2018
Figure 2.3 Global Biosimilar Market Restraints, 2018
Figure 4.1 Sandoz: Biosimilar Products Revenue Forecast ($bn), 2018-2028
Figure 4.2 Teva Pharmaceuticals: Biosimilar Products Revenue Forecast ($bn), 2018-2028
Figure 4.3 Mylan Pharmaceuticals: Biosimilar Products Revenue Forecast ($bn), 2018-2028
Figure 6.1 Biocon: Biosimilar Products Revenue Forecast ($bn), 2018-2028
Figure 9.1 SWOT Analysis of the Biosimilars Market, 2018
Figure 9.2 STEP Analysis of the Biosimilars Market, 2018




























