Industries > Pharma > Global Stem Cell Technologies and Applications Market 2019-2029
Global Stem Cell Technologies and Applications Market 2019-2029
Cancer, Cardiovascular, CNS, Other Disease Areas and Non-Therapeutic Applications
The global stem cell technologies and applications market is estimated to have reached $14bn in 2018 and is expected to grow at a CAGR of 12.3% in the first half of the forecast period.
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in this sector.
In this brand new 292-page report you will receive 110 charts– all unavailable elsewhere.
The 292-page report provides clear detailed insight into the global stem cell technologies and applications market. Discover the key drivers and challenges affecting the market.
By ordering and reading our brand-new report today you stay better informed and ready to act.
Report Scope
• Global Stem Cell Technologies and Applications Market forecasts from 2019-2029
• Global Stem Cell Technologies and Applications submarket forecasts from 2019-2029:
• Cancer treatment
• Cardiovascular therapy
• CNS
• Other therapies
• Non-therapeutic applications
• Individual revenue forecast to 2029 for selected top products:
• MSC-100-IV (Mesoblast)
• Hearticellgram-AMI (Pharmicell)
• CardioRel (Reliance Life Sciences)
• Osteocel Plus (NuVasive)
• Trinity Evolution and Elite (Orthofix)
• CARTISTEM (MEDIPOST)
• Analysis of the most promising pipeline therapies in each therapeutic segment
• Discussion on regulatory environments and developments in the US, Japan, Europe and other leading countries
• Analysis of what drives and restrains the market
• This study also discusses other influences on that field, including these:
• Haematopoietic stem cell transplantation (HSCT)
• Embryonic stem cells (ESCs), induced pluripotent adult (IPSCs) and parthenogenetic cells
• Uses for umbilical cord blood and related technologies, including cellular and blood banking
• Agents for osteogenesis and treating autoimmune conditions
• Applications in cell-based assays, diagnostics and drug development.
• Key questions answered by this report:
• What is the size of the total stem cells market, and how will the market evolve between 2019 and 2029
• How will the main segments within the overall stem cells market develop between 2019-2029
• What are the main drivers and restraints that will shape the overall stem cells market, and its individual segments over the next ten years?
• What is the state of stem cell research in the different therapeutic segments?
• What are some of the most prominent companies within this space, and what are their latest developments?
• What are the most promising pipeline therapies in each therapeutic segment?
• What are the revenue prospects for some of the stem cell therapies which have already been approved?
• What are the main strengths, weaknesses, opportunities and threats for the stem cell market?
• What are the main social, technological, economic and political factors that influence this market?
Visiongain’s study is intended for anyone requiring commercial analyses for the Global Stem Cell Technologies and Applications Market. You find data, trends and predictions.
Buy our report today Global Stem Cell Technologies and Applications Market Analysis : Cancer, Cardiovascular, CNS, Other Disease Areas and Non-Therapeutic Applications.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help sara.peerun@visiongain.com
1. Report Overview
1.1 Global Stem Cell Technologies and Applications Market Overview
1.2 Why you should Read This Report
1.3 How this Report Delivers
1.4 Key Questions Answered by This Report
1.5 Who is this Report for?
1.6 Research and Analysis Methods
1.7 Frequently Asked Questions (FAQ)
1.8 Some Associated Reports
1.9 About Visiongain
2. Introduction to Stem Cell Technologies and Applications
2.1 What are Stem Cells? Defining Characteristics
2.2 Timeline of Stem Cell Research
2.3 Embryonic Stem Cells (ESCs) Enter the Picture
2.4 Classifying Stem Cells by Potency
2.4.1 Post Fertilisation and Germ Layers
2.5 The Main Types of Stem Cells and Their Properties
2.6 iPSCs: The Benefits of ESCs, but Without the Downsides?
2.6.1 CIRM hPSC Repository Launches 300 iPSC Lines
2.7 Parthenogenetic Stem Cells: Scientific Background
2.7.1 Are they Ethical Alternatives to ESCs? What are the Commercial Applications?
2.8 Autologous Versus Allogeneic Stem Cells
2.8.1 Are Universal Stem Cell Products Possible?
2.9 Phases of Clinical Trials
2.10 Scope of this Report
3. International Developments and Stem Cell Regulatory Environments
3.1 Regulatory Environment in the US
3.1.1 Dynamic Biotechnology Sector and Liberal Stance on Patents
3.1.2 Understanding ‘Homologous’ Use
3.1.3 Controversy Surrounding ESC Research
3.1.4 NIH ESC Stem Cell Registry: Has Made Good Progress, but not enough for its Critics
3.1.5 CIRM and NIH Stem Cell Funding into Various Channels
3.1.6 FDA vs Regenerative Sciences
3.1.7 Are Stem Cells Clinics the ‘Wild West’ of Medicine?
3.1.8 Stem Cell Clinics Using Loopholes to Avoid Oversight?
3.1.9 FDA Releases Three New Draft Guidances, but is it Doing Enough?
3.2 Regulatory Environment in Europe
3.2.1 Unified Overarching System of Regulation Across EU
3.2.2 Difference between EU Member States Regarding hESC Research
3.2.3 European Court of Justice Performs a U-Turn and Clears Path towards Stem Cell Patents
3.2.4 The UK: A Leader in European Stem Cell Research
3.3 Regulatory Environment in Japan
3.3.1 Liberal Stance on hESC but with Excessive Bureaucracy?
3.3.2 Japan: A Pioneer in iPSC Research, Although First In-Human Trial has been suspended
3.3.3 The STAP Cell Events: Excitement Dashed
3.3.3.1 Riken Concludes Investigation
3.3.4 Government Committed to Regenerative Medicines and iPSC Research
3.3.5 Japan Becomes Very Attractive Market for Stem Cell Companies due to Favourable Regulatory Changes
3.4 Regulatory Environment in South Korea
3.4.1 Highs and Lows with Regard to hESC Research
3.4.2 Regulators are Quick to Approve Therapies, although this has Drawn Criticism
3.4.3 Tracheal Transplant Patient Dies
3.4.4 New changes a requirement for the current research and development in HESC
3.5 Chinese Regulatory Environment
3.5.1 Liberal Regulation on Stem Cells and Very High Number of Stem Cell Clinical Trials Taking Place in the Country
3.5.2 Stem Cell Medical Tourism Very Popular
3.5.3 CFDA Releases New Stem Cell Draft Guidance
3.6 Israel Regulatory Environment: A Strong Presence in Stem Cell Research
3.7 Indian Regulatory Environment: Emerging Stem Cell Industry – in Need of more Regulation?
4. Stem Cell Technologies and Applications: World Market 2019-2029
4.1 Therapeutic Applications versus Non-Therapeutic Applications of Stem Cells
4.2 Regional Breakdown of the World Market
4.3 Market Segmentation
4.4 Stem Cell Technologies and Applications Market: Forecast 2019-2029
4.5 Stem Cell Technologies and Applications Market: Forecast by Segment 2019-2029
4.6 How Will Segmental Market Shares Change to 2029?
4.7 Stem Cell Technologies and Applications Market: Drivers and Restraints
5. Stem Cell Cancer Therapeutics Segment 2019-2029
5.1 Stem Cell Cancer Therapeutics: Overview and Current Status
5.2 Stem Cell Cancer Therapeutics: Market Forecast 2019-2029
5.3 Haematopoietic Stem Cell Transplantation (HCST)
5.3.1 An Established Treatment for Haematological Cancers
5.3.2 Autologous HSCT: Dominant Form of HSCT
5.3.3 Autologous Versus Allogeneic HSCT: Advantages and Disadvantages
5.3.4 GvHD: The Major Issue for HSCT
5.3.5 Strengths and Weaknesses of the Three Major Sources of HSCs
5.3.6 What is Cord Blood?
5.3.6.1 Cord Blood Use is Rising in HSCT
5.3.7 Number of HSCT Operations Performed Worldwide, Split by Autologous Versus Allogeneic HSCT, 2015-2017
5.3.7.1 Numbers of HSCT Operations Performed in Europe, Split by Donor Type, 2011-2017
5.3.8 HSCT Costs and Medicare Coverage
5.3.9 The Indications which can be treated with HSCT: Haematological Malignancies are the Major Indications
5.3.10 HSCT has the Potential to Address Multiple Cancers, which represent a $20bn+ Market
5.4 From Procedures to Products: Cord Blood Stem Cell Approvals
5.4.1 Hemacord (New York Blood Center): The First FDA-Approved Cord Blood Product
5.4.1.1 Hemacord Awarded Prix Galien ‘Best Biotechnology Product’ Award
5.4.2 HPC, Cord Blood (ClinImmune Labs / University of Colorado Blood Bank)
5.4.3 Ducord (Duke University School of Medicine)
5.4.4 Allocord (SSM Cardinal Glennon Children’s Medical Center)
5.4.5 HPC, Cord Blood BLA 125432 (LifeSouth Community Blood Centers)
5.4.6 CLEVECORD
5.4.7 HPC, Cord Blood (BLA 125585)
5.5 Stem Cell Cancer Therapeutics Pipeline
5.5.1 Agenmestencel-T (Apceth)
5.5.2 CLT-008 and CLT-009 (Cellerant Therapeutics): Company Receives $47.5m Grant from US Government
5.5.3 ProHema (Fate Therapeutics)
5.5.3.1 ProTmune
5.5.4 StemEx and NiCord (Gamida Cell)
5.5.4.1 StemEx Shows Improved Survival at 100 Days – but FDA Wants Another Clinical Trial
5.5.4.2 Copper Chelator Based Technology
5.5.4.3 NiCord
5.5.4.4 NiCord: The Company Aiming for a “Paradigm Shift” in Treatment Practice, Receives Orphan Drug Designation
5.5.4.5 NiCord: Gamida Cell Reaches Agreement on Phase 3 Design Outline with the FDA and EMA
5.5.4.6 NAM Technology Platform
5.5.4.7 Novartis Chooses not to buy Gamida Cell
5.5.5 MSC-100-IV (Previously Known as Prochymal) and Unnamed MPC-Expanded Cord Blood Product (Mesoblast)
5.5.5.1 MSC-100-IV: World’s First Approved Stem Cell Drug Outside of South Korea
5.5.5.2 MSC-100-IV: Culture Expanded and Able to Treat GvHD
5.5.5.3 An Important Role to Play in the Future of HSCT
5.5.5.4 Wins Approval in Japan, through Partner JCR Pharmaceuticals, and Phase 3 Trial will Support US BLA
5.5.5.5 MSC-100-IV / Temcell HS Injection Revenue Forecast 2019-2029
5.5.5.6 MPC-Expanded Cord Blood Product in a Phase 3 Trial for Haematological Malignancies
5.5.6 HSC835 (Novartis)
5.5.6.1 Novartis Enters Partnership Agreement with Regenerex to Develop Novel Cell-Based Therapies
5.5.6.2 Collaborations with Intellia Therapeutics and Caribou for CRISPR and HSCs
5.5.6.3 Collaborations with Gamida Cell, Israel
5.5.7 Ancillary Products for the HSCT Setting
5.5.8 Targeting of Cancer Stem Cells (CSCs)
5.6 Stem Cell Cancer Therapeutics: Drivers and Restraints 2019-2029
6. Stem Cell Cardiovascular Therapeutics Segment 2019-2029
6.1 Stem Cell Cardiovascular Therapeutics: Overview and Current Status
6.2 Stem Cell Cardiovascular Therapeutics: Market Forecast 2019-2029
6.3 Cardiovascular Diseases Make up a Large part of the Global Pharmaceutical Market
6.3.1 Stem Cell Treatments for Stroke: Touched Upon in This Chapter but mainly Covered under Central Nervous System Therapeutics
6.4 Stem Cell Cardiovascular Therapeutics on the Market
6.4.1 Hearticellgram-AMI (Pharmicell)
6.4.1.1 One of the First Approved Cardiovascular Stem Cell Treatments in the World
6.4.1.2 Hearticellgram-AMI Sales Estimates 2014-2017
6.4.1.3 Will Have Difficulty Getting Approved in Markets Outside of South Korea
6.4.1.4 Hearticellgram-AMI Revenue Forecast 2019-2029
6.4.2 CardioRel (Reliance Life Sciences)
6.4.2.1 Available on the Indian Market, but Does Data Back it up?
6.4.2.2 CardioRel Revenue Forecast 2019-2029
6.4.3 Cardiovascular and Cerebrovascular Conditions are the World’s Most Fatal Diseases
6.4.4 AMI, CLI and Stroke: Major Ischemic Disease Targets
6.5 The State of Stem Cell Research for Cardiovascular Diseases
6.5.1 Practical Advantages to Choosing the Heart as a Target for Stem Cell R&D
6.5.2 Defining Cardiovascular Stem Cells: Scientific Context
6.6 The Mysterious Effects of Stem Cells in the Heart
6.6.1 MSCs in the Heart: Evidence to Support their Use, as well as Doubts over their Efficacy
6.6.2 Cardiosphere-Derived Cells, hESC-Derived Cardiomyocytes and C-kit Cells
6.7 Stem Cell Cardiovascular Therapeutics Pipeline
6.7.1 Ixmyelocel-T (Vericel Corporation)
6.7.1.1 Vericel Shifts Focus Away from CLI and Towards DCM
6.7.1.2 Currently it has obtained RMAT designation
6.7.2 Alecmestencel-T (Apceth)
6.7.3 Arteriocyte: Using its ‘Magellan System’ to Treat CLI
6.7.4 MultiStem (Athersys) for Myocardial Infarction and Stroke
6.7.4.1 Updates from Athersys Regarding Trial Status: Did MultiStem Fail in Stroke, or was it just Being Assessed in the Wrong Treatment Window?
6.7.5 Baxter: Phase 3 CD34+ Stem Cell Therapy for Refractory Angina
6.7.6 CardiAMP (BioCardia) for Heart Failure: Scheduled to Finally Begin Phase 3 in January 2017
6.7.7 MyoCell (U.S. Stem Cell, Inc. – Formerly Bioheart): Autologous ‘Muscle Stem Cell’ Therapy
6.7.7.1 MyoCell: Is the Company Still Trying to Secure Funds to Restart the Phase 2/3 MARVEL Trial?
6.7.7.2 MyoCell: MIRROR Trial on Hold
6.7.7.3 MyoCell SDF-1: Improved Version of MyoCell
6.7.7.4 MyoCell SDF-1 Phase 1 Trial (Regen): Another Trial Put on Hold
6.7.8 CAP-1001 and CAP-1002 (Carpricor): Cardiospehere-Derived Cells for Heart Repair
6.7.9 Ceylad (Formerly Cardio3): Proprietary Cardiopoiesis Technology Platform - Can be Applied to Various Stem Cell Sources
6.7.9.1 C-Cure: Stem Cell Therapy for the Treatment of Heart Failure
6.7.9.2 C-Cure: Two Phase 3 Trials Under Way
6.7.10 Mesoblast: Mesenchymal Precursor Cells for Heart Failure and Myocardial Infarction
6.7.10.1 Rexlemestrocel-L for Congestive Heart Failure (CHF), Chief Executive Provides Update
6.7.10.2 CEP-41750: Promising Phase 2 Results, More Effective in a Particular Subset of Heart Failure Patients?
6.7.10.3 Phase 2 Trial of MPCs in Acute Myocardial Infarction
6.7.11 Caladrius Biosciences: Will not Continue Development of CLBS10, the Company’s Former Lead Candidate
6.7.11.1 CLBS12 for CLI: Due to Start Phase 2 in Japan in January 2016
6.7.12 Stemedica Cell Technologies: ‘Ischaemic-Tolerant’ Stem Cell Platform
6.7.12.1 Trials in Myocardial Infarction and Stroke
6.7.13 Gemacell and Cryocell (Human Stem Cells Institute): Awaiting Important Russian Legislation to be Passed before Development Can Continue
6.8 Stem Cell Cardiovascular Therapeutics: Drivers and Restraints 2019-2029
7. Stem Cell Central Nervous System Therapeutics Segment 2019-2029
7.1 Stem Cell Central Nervous System Therapeutics: Overview and Current Status
7.2 Stem Cell Central Nervous System Therapeutics: Market Forecast 2019-2029
7.3 The State of Stem Cell Research in CNS Diseases
7.3.1 Stem Cells are the Best Hope for Many Serious CNS Conditions
7.3.2 Awaiting a First Breakthrough Approval
7.3.3 Human Neural Stem Cells (NSCs) Successfully Isolated
7.3.4 NSCs Reach the Clinic without Serious Controversy
7.3.5 CNS Disorders are a Major Focus for ESC Research
7.3.6 MSCs: Can Glial Cell and Astrocyte Formation Help Neurological Conditions?
7.4 Progress in Specific CNS Conditions
7.4.1 HSCT in MS: Could ‘Resetting’ the Immune System Treat MS?
7.4.1.1 Clinical Trials Involving HSCT in MS
7.4.1.2 Barriers to Overcome Before HSCT Could Become an Established Therapy for MS
7.4.2 ALS: Could this Rare Disease be the First Neurodegenerative Condition for Stem Cell Treatment?
7.4.3 Parkinson’s Disease: Field Recovering after Voluntary Moratorium on Stem Cell Research by Many Western Nations Ends
7.4.4 Dry Age-Related Macular Degeneration: A Major Unmet Need
7.5 Several Multi-Billion Dollar Markets are Open to Stem Cell Developers if they can Translate the Potential of Stem Cells into Therapies
7.6 Stem Cell Central Nervous System Therapeutics Pipeline
7.6.1 MA09-hRPE (Ocata Therapeutics): Stem Cells for Eye Diseases
7.6.1.1 Orphan Drug Status in both the US and EU
7.6.1.2 Current Clinical Trials in SMD
7.6.1.3 Early Signs of Efficacy
7.6.1.4 Has Moved into Phase 2 in AMD
7.6.2 NurOwn (BrainStorm Cell Therapeutics): Neurotrophic Factor -Releasing Stem Cells for ALS
7.6.2.1 Phase 2 Trial in 48 ALS Patients Underway
7.6.2.2 Phase 2a Results Demonstrate “Statistically Significant Effect”
7.6.3 International Stem Cell Corporation: Company Signals Intent to Start Phase 1/2a Study in PD Soon
7.6.4 Neuralstem: Allogeneic NSCs for Synaptic Repair and Neuroprotection
7.6.4.1 NSI-566 in ALS
7.6.4.2 NSI-566 in ALS: Encouraging Data from Completed Phase 2 Trial
7.6.4.3 NSI-566 for Chronic Spinal Cord Injury: Phase 1 Top-Line Data Expected Q4 2015
7.6.4.4 NSI-566 for Ischaemic Stroke: Phase 1/2 Trials Underway in China
7.6.4.5 Preclinical Investigation in 11 Other Indications
7.6.5 PF-05206388 (Pfizer and the London Project to Cure Blindness): Finally Begins Phase 1Trial, with first Successful Surgery
7.6.6 Q-Cells (Q Therapeutics): Phase 1/2 Trial Begins
7.6.7 ReNeuron: CTX Neural Cell Line and its Advantages
7.6.7.1 CTX for Stroke
7.6.7.2 CTX for Stroke: Long-Term PISCES Study Shows Promising Results
7.6.7.3 CTX for Stroke: Undergoing Phase 2, Using Highest Cell Dose from PISCES Study
7.6.8 SB623 (SanBio): Enters Phase 2 for Stroke, as well as for Traumatic Brain Injury
7.6.9 StemCells, Inc: A Leader in Neural Stem Cells
7.6.9.1 HuCNS-SC for Pelizaeus-Merzbacher Disease: Undergoing Second Phase 1 Trial
7.6.9.2 HuCNS-SC for NCL: Second Phase 1 Trial Withdrawn
7.6.9.3 HuCNS-SC for Spinal Cord Injury: Phase 2
7.6.9.4 HuCNS-SC for AMD: Top-Line Phase 1/2 Results Released
7.7 Stem Cell Central Nervous System Therapeutics: Drivers and Restraints 2019-2029
8. Stem Cell Therapeutics in Other Disease Areas 2019-2029
8.1 Stem Cell Therapeutics in Other Disease Areas: Overview and Current Status
8.2 Stem Cell Therapeutics in Other Disease Areas: Market Forecast 2019-2029
8.3 Stem Cell Therapeutics in Other Disease Areas: Therapies on the Market
8.3.1 Osteocel Plus (NuVasive)
8.3.1.1 Osteocel to Osteocel Plus: Passing Through Different Companies and Patent Disputes
8.3.1.2 The Leading Stem Cell Orthobiologic
8.3.1.3 Is it Better than Autograft?
8.3.1.4 Osteocel Plus Revenue Forecast 2019-2029
8.3.2 Trinity Evolution and Trinity Elite (Orthofix): Stem Cell Orthobiologics
8.3.2.1 Positive Results from New Trial in Foot and Ankle Procedures
8.3.2.2 Trinity Evolution and Elite Revenue 2010-2014
8.3.2.3 Trinity Evolution and Elite Revenue Forecast 2019-2029
8.3.3 CARTISTEM (MEDIPOST)
8.3.3.1 CARTISTEM: The World’s First Allogeneic Stem Cell Drug
8.3.3.2 CARTISTEM: Latest Updates Includes Results from Phase 3 Trial, Initiation of Phase 1/2 Trial in the US
8.3.3.3 CARTISTEM: Revenues 2014 –2016
8.3.3.4 CARTISTEM Revenue Forecast 2019-2029
8.3.4 Allostem (Allosource)
8.3.5 Map3 (RTI Surgical)
8.3.6 ReliNethra for Ocular Surface Damage (Reliance Life Sciences)
8.3.7 Cupistem (Anterogen)
8.4 Stem Cell Therapeutics for Other Diseases: Research Areas
8.4.1 HSCT for Orphan Diseases
8.4.2 Potential for Genetically Modified Stem Cells for HIV and Other Diseases?
8.4.3 Stem Cells Contribute to the Osteogenesis Process in Bone Repair
8.4.4 Stem Cells Gaining Increasing Importance in Bone Graft Market, but whether these are True ‘Stem Cell Products’ is Debatable
8.4.5 Stem Cells also have Potential in Autoimmune Disorders
8.4.6 Potential Cure for Diabetes?
8.4.6.1 Clinical Stage Programmes in Diabetes
8.4.7 Stem Cells in Active Liver Repair
8.4.8 Long-Term Possibilities
8.5 Stem Cell Therapeutics in Other Disease Areas: Pipeline
8.5.1 ALLO-ASC and ALLO-ASC-DFU (Anterogen)
8.5.2 MultiStem (Athersys): Fails Phase 2 Ulcerative Colitis, Still Undergoing Phase 1 as Immunomodulation Therapy after Liver Transplantation
8.5.3 BioTime: Multiple Subsidiaries Focused on Regenerative Medicine
8.5.3.1 AST-OPC1 and AST-VAC2 (Asterias Biotherapeutics)
8.5.3.2 OpRegen (Cell Cure Neurosciences)
8.5.3.3 Other BioTime Subsidiaries Related to Stem Cells: OncoCyte, ReCyte, ESI Bio, LifeMap Sciences Etc.
8.5.4 Calimmune: Dual Anti-HIV Gene Therapy via Stem Cells
8.5.5 (PDA-001 and PDA-002) Celgene Corporation
8.5.6 ReJoin (Cellular Biomedicine Group): Interim Results from the MSCs for Knee Osteoarthritis
8.5.7 PNEUMOSTEM (MEDIPOST)
8.5.7.1 NEUROSTEM (MEDIPOST): Undergoing Phase 1/2 Trial and Obtains Patents Around the Globe
8.5.8 Translational Biosciences: Set Up for the Sole Purpose of Conduction Stem Cell Clinical Trials, Five Phase 1/2 Trials Ongoing
8.5.9 Mesoblast: MPCs for Various Indications
8.5.9.1 MPC-300-IV in Diabetes
8.5.9.2 MPC-300-IV in Rheumatoid Arthritis
8.5.9.3 MPC-06-ID in Chronic Back Pain, Enters Phase 3
8.5.10 Pluristem Therapeutics: Uses PLX Cells to Secrete Therapeutic Proteins in Damaged Tissues
8.5.10.1 PLX-PAD: Phase 2 for Intermittent Claudication
8.5.10.2 PLX-PAD: Completes Phase 1/2 for Injured Gluteal Muscle
8.5.10.3 PLX-R18: In Preclinical Development for ARS
8.5.11 Regeneus: Adipose Derived MSC Therapies
8.5.11.1 Progenza Undergoing Phase 1 Trial
8.5.12 S-Evans Biosciences: Menstrual Stem Cells
8.5.13 TiGenix: Expanded Adipose-Derived Stem Cell Therapies
8.5.13.1 EASC Technology Platform
8.5.13.2 Cx601: Meets Primary Endpoint in Phase 3 Trial
8.5.13.4 Cx611 for Rheumatoid Arthritis and also Severe Sepsis
8.5.13.5 Cx621 – Positive Results from Phase 1 Trial, but Development on Hold
8.5.13.6 ChondroCelect Already on the Market – Although is More of a Cell Therapy Rather than a Stem Cell Therapy
8.5.14 VC-01 (ViaCyte)
8.6 Genetically Modified Stem Cell Therapies
8.6.1 BluebirdBio: Gene-Modified HSCs for Orphan Diseases
8.6.2 GSK: Stem Cell Gene Therapies for Rare Diseases, Including one which has been Submitted for Approval in Europe
9. Stem Cell Non-Therapeutic Applications 2019-2029
9.1 Stem Cell Non-Therapeutic Applications: Overview and Current Status
9.2 Stem Cell Non-Therapeutic Applications: Market Forecast 2019-2029
9.3 Stem Cell Banking: Growing Demand Worldwide
9.3.1 Impact of the current epidemic of Zika Virus and HIV in cord cell banking
9.3.2 Is there a Need for Stem Cell Banking as a Form of ‘Health Insurance’?
9.3.3 Stem Cell Banking Companies in the US and Around the World
9.3.4 Dental Stem Cell Banking: An Alternative to Cord Blood Banks?
9.4 Stem Cell Supply and Processing: iPSCs are the New Driver
9.4.1 Stem Cell Supply and Processing Companies
9.5 Stem Cell-Based Assays: Major Potential for Preclinical Screens
9.5.1 Advantages of Stem Cell-Based Assays
9.5.2 Stem Cell-Based Assay Companies
9.6 Research, Reagents, and Other Non-Therapeutic Stem Cell Activities
9.7 Drivers and Restraints
10. Qualitative Analysis of the Stem Cell Technologies and Applications Market 2019-2029
10.1 SWOT Analysis of the Stem Cell Technologies and Applications Market
10.2 Strengths
10.2.1 HSCT is already an Established Procedure
10.2.2 Approvals for Stem Cell Therapies
10.2.3 Relaxation of Regulatory Barriers
10.3 Weaknesses
10.3.1 Phase 3 Trials only make up a Small Fraction of the Currently Ongoing Clinical Trials
10.3.2 Uncertain Mechanisms of Action in Stem Cell Therapies
10.3.3 Regulatory and Reimbursement Concerns
10.3.4 The scare of a possible immune rejection after a stem cell transplant
10.4 Opportunities
10.4.1 Huge Potential in Unmet Clinical Needs
10.4.2 Opportunities in the Non-Therapeutic Uses of Stem Cells, including Cord Blood Banking and Cell-Based Assays
10.4.3 Interactions with Related Technologies will Offer new Opportunities
10.4.4 Genetic Modification of Stem Cells
10.5 Threats
10.5.1 Financial Risks and Under-Capitalisation of the Stem Cells Sector
10.5.2 Threat of Pipeline Failures
10.5.3 Long-Term Safety Concerns
10.6 STEP Analysis of the Stem Cell Technologies and Applications Market
10.7 Social Factors
10.7.1 Increasing Burden of Disease as World Population Ages
10.7.2 Biological Insurance through Stem Cell Banking
10.7.3 Stem Cell Tourism: Both an Opportunity and Threat to the Market
10.8 Technological Factors
10.8.1 Increasing Research Output
10.8.2 IPSC Advances
10.8.3 Greater Understanding of Stem Cell Differentiation
10.8.4 Interactions with Other Technologies
10.8.5 CRISPR: A Breakthrough in Genome Editing
10.9 Economic Factors
10.9.1 Grey Market for Stem Cell Therapies
10.9.2 Broad Changes in Pharma / Healthcare Markets
10.9.3 New Business Models are required due to the Unique Challenges Posed by these Non-Traditional Therapies
10.10 Political Factors
10.10.1 Controversies over Embryonic Stem Cell Research
10.10.2 Support from National Governments
10.10.3 Patient Pressure to Deregulate the Stem Cell Therapies Market
11. Conclusions
11.1 Stem Cell Technologies and Applications: An Emerging Market
11.2 Current Leading Segments and Regional Markets
11.3 World Stem cell Technologies and Applications Market Forecast 2019-2029
11.4 The Future of the Stem Cell Market
11.5 Concluding Remarks
Appendices
Glossary
Associated Reports
Visiongain Report Sales Order Form
About Visiongain
Visiongain report evaluation form
List of Tables
Table 2.1 Classifying Stem Cells by Potency
Table 2.2 The Different Germ Layers and Their Associated Types of Cells and Organs
Table 2.3 Main Types of Stem Cells and Their Properties
Table 2.4 Stem Cell Donor Terminology
Table 2.5 The Different Phases of Clinical Trials
Table 3.1 NIH Embryonic Stem Cell Registry: Stem Cell Lines by Status, 2016 and 2017
Table 3.2 Estimated and expected NIH spending in Stem cell research from 2015-2020 (expected) (in million USD)
Table 3.3 New FDA Draft Guidance’s
Table 3.4 StemGen’s Classifications of European Nations by Level of Permissiveness towards hESC research
Table 4.1 Stem Cell Technologies and Applications Market by Region: Revenues ($m), Market Shares (%), 2018
Table 4.2 Stem Cell Technologies and Applications Market by Segment: Revenues ($m), Market Shares (%), 2018
Table 4.3 Stem Cell Technologies and Applications Market Forecast: Revenues ($m), AGR (%), CAGR (%), 2018-2023
Table 4.4 Stem Cell Technologies and Applications Market Forecast: Revenues ($m), AGR (%), CAGR (%), 2024-2029
Table 5.1 Stem Cell Cancer Therapeutics Market Forecast: Revenues ($m), AGR (%), CAGR (%), 2018-2023
Table 5.2 Stem Cell Cancer Therapeutics Market Forecast: Revenues ($m), AGR (%), CAGR (%), 2024-2029
Table 5.3 Comparison of Bone Marrow, Peripheral Blood and Cord Blood for HSCT
Table 5.4 Estimated Number of HSCT Operations Performed Globally, by Donor Type: AGR (%), CAGR (%), 2015-2017
Table 5.5 European HSCT Operations: Number of Operations by Donor Type, 2011-2017
Table 5.6 Indications with Medicare Coverage for HSCT (both Autologous and Allogeneic)
Table 5.7 Malignancies and Other Haematological Diseases which may be Treated with HSCT
Table 5.8 HSCT-Addressable Cancers: Estimated Combined Incidence in the US, Japan and Western Europe (Number of Cases), CAGR (%), 2012-2024
Table 5.9 FDA-Approved Cord Blood-Derived Stem Cell Products for HSCT, 2018
Table 5.10 Past and Present ProHema (Fate Therapeutics) Clinical Trials
Table 5.11 MSC-100-IV / Temcell HS Forecast (Mesoblast / JCR Pharmaceuticals): Revenues ($m), AGR (%), CAGR (%), 2018-2023
Table 5.12 MSC-100-IV / Temcell HS Forecast (Mesoblast / JCR Pharmaceuticals): Revenues ($m), AGR (%), CAGR (%), 2024-2029
Table 5.13 HSC835 (Novartis) Ongoing Clinical Trials, 2017
Table 6.1 Stem Cell Cardiovascular Therapeutics Market Forecast: Revenues ($m), AGR (%), CAGR (%), 2018-2023
Table 6.2 Stem Cell Cardiovascular Therapeutics Market Forecast: Revenues ($m), AGR (%), CAGR (%), 2024-2029
Table 6.3 Global Cardiovascular Disease Market by Drug Class: Revenue($bn), 2018
Table 6.4 Hearticellgram-AMI (Pharmicell) Sales Estimates: Revenue ($m), AGR (%), 2014-2017
Table 6.5 Hearticellgram-AMI Forecast (Pharmicell): Revenues ($m), AGR (%), CAGR (%), 2018-2023
Table 6.6 Hearticellgram-AMI Forecast (Pharmicell): Revenues ($m), AGR (%), CAGR (%), 2024-2029
Table 6.7 CardioRel (Reliance Life Sciences) Forecast: Revenues ($m), AGR (%), CAGR (%), 2018-2023
Table 6.8 CardioRel (Reliance Life Sciences) Forecast: Revenues ($m), AGR (%), CAGR (%), 2024-2029
Table 6.9 Projected Global Deaths from Ischaemic Heart Disease and Stroke (millions), % of Total Deaths, Deaths Per 100,000 Population, 2015 and 2030
Table 6.10 Advantages, disadvantages and different clinical trials that are ongoing
Table 6.11 Past and Present MultiStem (Athersys) Clinical Trials, 2018
Table 6.12 Ongoing Stemedica Cell Technologies Clinical Trials in Cardiovascular Indications, 2018
Table 7.1 Stem Cell CNS Therapeutics Market Forecast: Revenues ($m), AGR (%), CAGR (%), 2018-2023
Table 7.2 Stem Cell CNS Therapeutics Market Forecast: Revenues ($m), AGR (%), CAGR (%), 2024-2029
Table 7.3 Ongoing MA09-hRPE (Ocata Therapeutics) Clinical Trials in SMD, 2018
Table 7.4 Ongoing MA09-hRPE (Ocata Therapeutics) Clinical Trials in AMD
Table 8.1 Stem Cell Therapeutics in Other Disease Areas Market Forecast: Revenues ($m), AGR (%), CAGR (%), 2018-2023
Table 8.2 Stem Cell Therapeutics in Other Disease Areas Market Forecast: Revenues ($m), AGR (%), CAGR (%), 2024-2029
Table 8.3 Osteocel Plus Forecast (NuVasive): Revenues ($m), AGR (%), CAGR (%), 2018-2023
Table 8.4 Osteocel Plus Forecast (NuVasive): Revenues ($m), AGR (%), CAGR (%), 2024-2029
Table 8.5 Trinity Evolution and Elite (Orthofix): Revenues ($m), AGR (%), CAGR (%), 2010-2014
Table 8.6 Trinity Evolution and Elite (Orthofix) Forecast: Revenues ($m), AGR (%), CAGR (%), 2018-2023
Table 8.7 Trinity Evolution and Elite (Orthofix) Forecast: Revenues ($m), AGR (%), CAGR (%), 2024-2029
Table 8.8 CARTISTEM Revenues: Revenue ($m), AGR (%), 2014-2016
Table 8.9 CARTISTEM (MEDIPOST) Forecast: Revenues ($m), AGR (%), CAGR (%), 2018-2023
Table 8.10 CARTISTEM (MEDIPOST) Forecast: Revenues ($m), AGR (%), CAGR (%), 2024-2029
Table 8.11 Non-Oncologic Diseases which can be Treated with HSCT
Table 8.12 Processes and Mechanisms of Bone Repair
Table 8.13 Anterogen Clinical Trials, 2018
Table 8.14 PDA-002 (Celgene Corporation) Current Clinical Trials
Table 8.15 PNEUMOSTEM (MEDIPOST) Past and Present Clinical Trials
Table 8.16 Translational Biosciences Currently Open Clinical Trials, 2015
Table 8.17 S-Evans Biosciences Clinical Trials, 2015
Table 9.1 Stem Cell Non-Therapeutic Applications Market Forecast: Revenues ($m), AGR (%), CAGR (%), 2018-2023
Table 9.2 Stem Cell Non-Therapeutic Applications Market Forecast: Revenues ($m), AGR (%), CAGR (%), 2024-2029
Table 9.3 Selected US Stem Cell Banks, 2018
Table 9.4 Selected Non-US Stem Cell Banks, 2017
Table 9.5. Prominent Dental Stem cell banks globally
Table 9.6 Selected Stem Cell Supply and Processing Companies
Table 9.7 Selected Companies with Involvement in Stem Cell-Based Assays, 2017
Table 10.1 World 65+ Population Forecast: Population (m), 2015-2050
Table 10.2 World 65+ Population Forecast: Population (m), CAGR (%), 2015-2050
List of Figures
Figure 1.1 Global Stem Cell Technologies and Applications Market Segmentation Overview, 2018
Figure 2.1 Timeline of Stem Cell Research
Figure 3.1 NIH Embryonic Stem Cell Registry: Stem Cell Lines by Status, 2017
Figure 3.2 NIH Spending for stem cell research by year (in USD billions)
Figure 3.3 CIRM Funding by Disease Area, Percentage (%), 2017
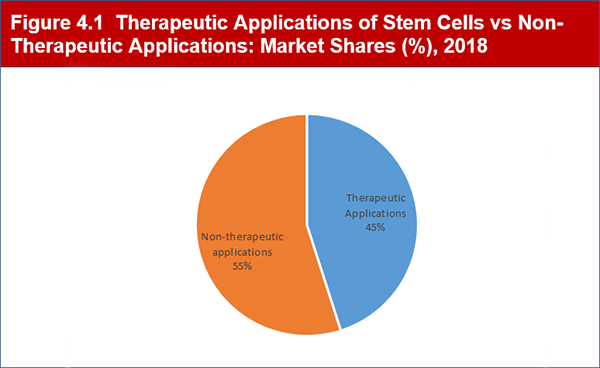
Figure 4.1 Therapeutic Applications of Stem Cells vs Non-Therapeutic Applications: Market Shares (%), 2018
Figure 4.2 Therapeutic Applications of Stem Cells vs Non-Therapeutic Applications: Market Shares (%), 2029
Figure 4.3 Stem Cell Technologies and Applications Market by Region: Market Shares (%), 2018
Figure 4.4 Stem Cell Technologies and Applications Market by Segment: Market Shares (%), 2018
Figure 4.5 Stem Cell Technologies and Applications Market Forecast: Revenues ($m), 2019-2029
Figure 4.6 Stem Cell Technologies and Applications Market Forecast by Segment: Revenue ($m), 2019-2029
Figure 4.7 Stem Cell Technologies and Applications Market: Market Shares (%) by Segment, 2023
Figure 4.8 Stem Cell Technologies and Applications Market: Market Shares (%) by Segment, 2029
Figure 4.9 Stem Cell Technologies and Applications Market: Drivers and Restraints
Figure 5.1 Estimated Proportions of Global HSCT Operations by Donor Type, 2017
Figure 5.2 European HSCT Operations by Donor Type, 2011-2017
Figure 5.3 Estimated New Cases of Leukemia, Lymphoma and Myeloma in 2017
Figure 5.4 Estimated new cases of leukemia diagnosed in 2017
Figure 5.5 HSCT-Addressable Cancers: Estimated Combined Incidence in the US, Japan and Western Europe (Number of Cases), 2012-2024
Figure 5.6 Most Common Diseases Treated by Cord Blood from LifeCord: Percentage (%) 2017
Figure 5.7 MSC-100-IV / Temcell HS Forecast (Mesoblast / JCR Pharmaceuticals): Revenue ($m), 2019-2029
Figure 5.8 Stem Cell Cancer Therapeutics Market: Drivers and Restraints
Figure 6.1 Stem Cell Cardiovascular Therapeutics Market Forecast: Revenue ($m), 2019-2029
Figure 6.2 Global Cardiovascular Disease Market by Drug Class: Market Shares (%), 2018
Figure 6.3 Hearticellgram-AMI (Pharmicell) Sales Estimates: Revenue ($m), 2014-2017
Figure 6.4 Hearticellgram-AMI (Pharmicell) Forecast: Revenue ($m), 2019-2029
Figure 6.5 CardioRel (Reliance Life Sciences) Forecast: Revenue ($m), 2019-2029
Figure 6.6 Projected Global Deaths from Ischaemic Heart Disease and Stroke (millions ), 2015 and 2030
Figure 6.7 Stem Cell Cardiovascular Therapeutics Market: Drivers and Restraints, 2019-2029
Figure 7.1 Stem Cell CNS Therapeutics Market Forecast: Revenue ($m), 2019-2029
Figure 7.2 Stem Cell CNS Therapeutics Market: Drivers and Restraints
Figure 8.1 Stem Cell Therapeutics in Other Disease Areas Market Forecast: Revenues ($m) 2019-2029
Figure 8.2 Osteocel Plus (NuVasive) Forecast: Revenue ($m), 2019-2029
Figure 8.3 Trinity Evolution and Elite (Orthofix): Revenue ($m), 2010-2014
Figure 8.4 Trinity Evolution and Elite (Orthofix): Revenue ($m), 2019-2029
Figure 8.5 CARTISTEM (MEDIPOST) Forecast: Revenues ($m) 2019-2029
Figure 9.1 Stem Cell Non-Therapeutic Applications Market Forecast: Revenue ($m), 2019-2029
Figure 9.2 Stem Cell Non-Therapeutic Applications Market: Drivers and Restraints
Figure 10.1 SWOT Analysis of the Stem Cell Technologies and Applications Market
Figure 10.2 STEP Analysis of the Stem Cell Technologies and Applications Market
Figure 10.3 World 65+ Population Forecast: Population (m), 2015-2050
Figure 12.1 Stem Cell Technologies and Applications Market Forecast, split by Therapeutic vs Non-Therapeutic Applications: Revenue ($m), 2019-2029
Aastrom Biosciences
Advanced Cell Technology
Alder Biopharmaceuticals
Alkem
AllCells
Allergan
AlloSource
Americord
Americord
Amgen
Amgen
Angiocrine Bioscience
Angiocrine Bioscience
Anterogen
Anterogen
Apceth
Arteriocyte
Arteriocyte
Associated Press
Astellas Pharma
Asterias Biotherapeutics
Asterias Biotherapeutics
Athersys
Athersys
Auriga Ventures
Axiogenesis
Axiogenesis
Bank a Tooth
Baxter
Baxter Healthcare
BaYi Brain Hospital
BioCardia
BioCardia
BioE
BioE
BioEden
BioEden
Biogenea-Cellgenea
Bioheart, Inc.
Biologic Therapies
BioMet Orthopedics
Biosciences
BioTechnology
BioTime
BioTime Asia
Blackstone
Blackstone Medical
Bloodworks
Bluebird Bio
Brain Somatic Mosaicism
BrainStorm Cell Therapeutics
Caladrius Biosciences
California Institute for Regenerative Medicine (CIRM)
Calimmune
Capricor
Cardio3
Caribou
Carolinas Cord Blood Bank (CCBB)
Casey Eye Institute
Cedars-Sinai Heart Institute
Celgene
Cell Cure Neurosciences
Cell Research Groups
Cell Therapy Catapult
Cellartis
CellCentric
Cellectis
Cellerant Therapeutics
Cellerix
CELLTREE
Cellular Biomedicine Group (CBMG)
Cellular Dynamics International
Center for International Blood and Marrow Transplant Research (CIBMTR)
Centers for Medicare & Medicaid Services
Cephalon
Cesca Therapeutics
Ceylad
China Cord Blood Corporation
China Cord Blood Corporation
China Regenerative Medicine International Limited (CRMI)
Chugai Pharmaceutical Co. Ltd.
CJ CheilJedang
Clal Biotechnologies Industries
Cleveland Biolabs
Cleveland Cord Blood Center
ClinImmune Labs
Cochrane
Cognate BioServices
Cook General BioTechnology
Cord Blood America
Cordlife Group
CordVida
CRISPR Therapeutics
Cryo-Cell International
CryoCord
CryoHaldco
Cryonix CJSC
Cryo-Save
CryoViva
CXR Biosciences
Cytori Therapeutics
Denali Ventures
Dendreon
Dong-A Pharmaceuticals Co.
Dynamics
Elbit Imaging
Elbit Medical Technologies
Eli Lilly
EpiStem
ESI BIO
EuroStemCell
Fate Therapeutics
FDS Pharma
Fondazione Centro San Raffaele
Fondazione Telethon
Forbion Capital Partners
Forticell Bioscience
Future Health Biobank
Gamida Cell
Gemabank
Gene Cell International
Genetico
Genetrix Group
Geron Corporation
Gilead
GlobalStem
Globocan
GSK
Howard Hughes Medical Institute
Human Stem Cells Institute
ImmunoCellular Therapeutics
Immunovative Therapies
Indian Department of Biotechnology
Insception Biosciences
Intellia Therapeutics
IntelliCell Biosciences
IPS Academia Japan
Isar Medical Centre
Israel Healthcare Venture
Japan Institute of Biomedical Research.
JCR Pharmaceuticals
JingYuan Bio.
Johnson & Johnson
Kirby Institute
Lifebank Cryogenics
LifebankUSA
LifeCord
LifeMap Sciences
LifeMap Solutions
LifeSouth Community Blood Centers Inc
Lonza
Maxcyte
Mayo Clinic
MedCell Bioscience
MEDIPOST
Medistem Panama
Medistem Panama Inc.
Medtronic
Mesoblast
Miltenyi Biotec
National Dental Pulp laboratory
NeoStem
Neuralstem
New England Cord Blood Bank
New York Blood Center
Novartis
NovoCell
NurOwn
Nuvasive
Ocata Therapeutics
OncoCyte
Opexa Therapeutics
OrLife Bio
Orthofix
Osiris Therapeutics
Pahrump Valley Times
Pfizer
Pharmicell
Pharmsynthez
Plasticell
Pluristem Therapeutics
Polyphor
Precious Cell Group
Prix Galien
PsychENCODE
Pulp Laboratory
Q Therapeutics
Quest Biomedical
ReCyte Therapeutics
Ree Labs
Regenerative Sciences
Regenerex
Regeneron
Regeneus
Reliance Group
Reliance Life Sciences
ReNeuron
RepliCel Life Sciences
Reprobank
ReproCELL
RIKEN Center for Developmental Biology
Roche
Roslin Cellab
RTI Biologics
RTI Surgical
RUSNANO Corporation
RVC OJSC
SanBio
Sangamo BioSciences
Sanofi
S-Evans Biosciences
Spinesmith Partners
SSM Cardinal Glennon
SSM Cardinal Glennon Children's Medical Center
Stem Cell Bank
Stemade
Stemcell Technologies
StemCore
StemCyte
Stemedica Cell Technologies
StemGen
StemImmune Inc
Stemina Biomarker Discovery
StemSave
Store-a-tooth
SynBio
Systems
Takara Bio
Takeda
TAP Biosystems
Teva
The Zon Laboratory
TiGenix
Tong Yuan Stem Cell
Tooth Bank
TrakCel
Transcell Biologics Pvt Ltd.
Translational Biosciences
U.S. Stem Cell, Inc
UCL Business PLC
UCL Institute of Ophthalmology
UCLA
uniQure
US Stem Cell, Inc.
Valeant
VAULT Sc Inc.
Vericel Corporation
Vericel Corporation
Vesta Therapeutics
ViaCord
ViaCyte
ViroMed
Vita34
Vitro Biopharma
WA Optimum Health Care
WHO
List of Organisations Mentioned in the Report
American Academy of Neurology
American Academy of Pediatrics (AAP)
American Neurological Associates Annual Meeting
American Society of Blood and Marrow Transplantation.
Americas Committee for Treatment and Research in Multiple Sclerosis
Boston Children’s Hospital
Cha General Hospital
China Food and Drug Administration (CFDA)
Chinese Academy of Sciences
Chinese Ministry of Health
Chinese Ministry of Science and Technology
Committee for Medicinal Products for Human Use (CHMP)
Consortium of Multiple Sclerosis Centers
Cord Blood Registry
Duke University School of Medicine
EMA
European Court of Human Rights
European Court of Justice
European Group for Blood and Marrow Transplantation (EBMT)
European Haematology Association
Federal D.C. Court of Appeals
Harvard Business School
Harvard Stem Cell Institute
Harvard University
Human Fertilisation and Embryology Authority (HFEA)
Human Tissue Authority
International Society for Stem Cell Research (ISSCR)
International Stem Cell Corporation (ISCO)
International Stemcell Services
Israel Stem Cell Society
Japan’s Ministry of Economy, Trade and Industry (METI)
Japanese Health Ministry
Japanese Ministry of Labour and Welfare (MHLW)
Japanese Pharmaceuticals and Medical Devices Agency
Karolinska Institute
Korean Food and Drug Administration
Korean MFDS (Ministry of Food and Drug Safety)
Massachusetts General Hospital
McMaster University
Musculoskeletal Transplant Foundation (MTF)
New York Heart Association
NHS Blood and Transplant Authority
Northwestern University
Novartis Research Foundation
Oregon Health and Science University
Psychiatric Gene Networks
Queen Mary University of London
Tel Aviv University
The European Commission
The Marcus Foundation
UK Care Quality Commission
UK Department of Health
UK Department of Health and the Medical Research Council
UK Home Office
UK Medicines and Healthcare Products Regulatory Agency (MHRA)
UK National Institute for Health Research
UK Regenerative Medicine Platform
United Nation’s Department of Economic and Social Affairs
University of California
University of Colorado Anschutz Medical Campus (AMC)
University of Colorado Cord Blood Bank
University of Kyoto
University of Massachusetts
University of Washington
University of Western Ontario
University of Wisconsin Alumni Research Foundation
US Congress
US District Court for the District of Columbia
US FDA
US National Cord Blood Program
US National Institute of Allergy and Infectious Diseases (NIAID)
US National Institute of Health (NIH)
US Patent and Trademark Office
US Supreme Court
Yale University
Download sample pages
Complete the form below to download your free sample pages for Global Stem Cell Technologies and Applications Market 2019-2029
Related reports
-

3D Printing for Healthcare Market Report 2019-2029
The latest report from business intelligence provider Visiongain offers a comprehensive analysis of the global 3D printing for the Healthcare...
Full DetailsPublished: 02 August 2019 -

Global Vaccines Sales Market Forecast 2019-2029
The global vaccines market is estimated at $41bn in 2018 and is expected to grow at a CAGR of 10.9%...
Full DetailsPublished: 25 June 2019 -

Global Biosimilars and Follow-On Biologics Market 2019-2029
The global biosimilars and follow-on biologics market is estimated to have reached $10.7bn in 2018 and expected to grow at...
Full DetailsPublished: 21 March 2019 -

Collagen Market Report 2019-2029
The collagen market is estimated to have reached $3.8bn in 2018 and expected to grow at a CAGR of 8.10%...Full DetailsPublished: 08 April 2019 -

Pharmaceutical Contract Manufacturing Market 2019-2029
The pharmaceutical contract manufacturing market is expected to grow at a CAGR of 5.7% in the first half of the...Full DetailsPublished: 19 August 2019 -

Global Graft versus Host Disease (GVHD) Market 2019-2029
The global graft versus host disease market is expected to grow at a CAGR of 7% from 2016-2021 and CAGR...
Full DetailsPublished: 01 January 1970 -

Biologics Market Trends and Forecasts 2019-2029
The global biologics market is estimated to reach $266bn in 2024. The market is expected to grow at a CAGR...
Full DetailsPublished: 28 August 2019 -

Advanced Wound Care Market Forecast 2019-2029
The global advanced wound care market was valued at $9.2bn in 2018. The largest segment of the advanced wound care...
Full DetailsPublished: 30 August 2019 -

Biological Drug API Manufacturing Services World Industry and Market Forecast to 2029
The biological drug API manufacturing market is estimated to grow at a CAGR of 9.5% in the first half of...Full DetailsPublished: 07 November 2019 -

Global Drug Discovery Outsourcing Market Forecast to 2028
The global drug discovery outsourcing market is estimated to have reach $22.69bn in 2018, dominated by the chemistry services segment....
Full DetailsPublished: 16 April 2019
Download sample pages
Complete the form below to download your free sample pages for Global Stem Cell Technologies and Applications Market 2019-2029
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Latest Pharma news
Visiongain Publishes Cell Therapy Technologies Market Report 2024-2034
The cell therapy technologies market is estimated at US$7,041.3 million in 2024 and is projected to grow at a CAGR of 10.7% during the forecast period 2024-2034.
18 April 2024
Visiongain Publishes Automation in Biopharma Industry Market Report 2024-2034
The global Automation in Biopharma Industry market is estimated at US$1,954.3 million in 2024 and is projected to grow at a CAGR of 7% during the forecast period 2024-2034.
17 April 2024
Visiongain Publishes Anti-obesity Drugs Market Report 2024-2034
The global Anti-obesity Drugs market is estimated at US$11,540.2 million in 2024 and is expected to register a CAGR of 21.2% from 2024 to 2034.
12 April 2024
Visiongain Publishes Inflammatory Bowel Diseases (IBD) Drugs Market Report 2024-2034
The global Inflammatory Bowel Diseases (IBD) Drugs market was valued at US$27.53 billion in 2023 and is projected to grow at a CAGR of 6.2% during the forecast period 2024-2034.
11 April 2024

















