Industries > Pharma > Decentralized Clinical Trials Market Report 2022-2032
Decentralized Clinical Trials Market Report 2022-2032
Forecasts by Study Design (Interventional Study Design, Observational Study Design, Expanded Access Study Design), by Indication (Oncology, Cardiovascular, Immunology, Respiratory, Others) by End-user (Pharmaceutical & Biopharmaceutical Companies, CROs, Others) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Recovery Scenarios
According to Visiongain analysis, global decentralized clinical trials market was valued at US$xx million in 2021 and is expected to grow at a CAGR of xx% during the forecast period 2022-2032 to reach US$xx million by 2032.
This report includes data analysis and invaluable insight into how COVID-19 will affect your industry. Access this report today.
To access the data contained in this document please email contactus@visiongain.com
Key Questions Answered by this Report:
• What is the current size of the overall global decentralized clinical trials market? How much will this market be worth from 2022 to 2032?
• What are the main drivers and restraints that will shape the overall decentralized clinical trials market over the next ten years?
• What are the main segments within the overall decentralized clinical trials market?
• How much will each of these segments be worth for the period 2022 to 2032?
• How will the composition of the market change during that time, and why?
• What factors will affect that industry and market over the next ten years?
• What are the largest national markets for the world decentralized clinical trials?
• What is their current status and how will they develop over the next ten years?
• What are their revenue potentials to 2032?
• How will political and regulatory forces influence regional markets?
• How will market shares of the leading national markets change by 2032, and which geographical region will lead the market in 2032?
• Who are the leading companies and what are their activities, results, developments and prospects?
• What are the leading decentralized clinical trials? What are their revenues and latest developments?
• What are some of the most prominent decentralized clinical trials currently in development?
• What are the main trends that will affect the world decentralized clinical trials market between 2022 and 2032?
• What are the main strengths, weaknesses, opportunities and threats for the market?
• What are the social, technological, economic and political influences that will shape that industry over the next ten years?
• How will the global decentralized clinical trials market evolve over the forecasted period, 2022 to 2032?
• What will be the main commercial drivers for the market from 2022 to 2032?
• How will market shares of prominent national markets change from 2022, and which countries will lead the market in 2032, achieving highest revenues and fastest growth?
• How will that industry evolve between 2022 and 2032, especially in R&D?
Need industry data? Please contact us today.
Advantages of decentralised trials is boosting the growth of global market. Find out why.
Decentralization has broadened trial access to reach a bigger number and perhaps a more diverse pool of patients, as 70 percent of eligible participants who often live more than two hours from trial sites. These various options enable a wide range of Decentralized and hybrid clinical-trial designs. In its most complete form, a trial can be totally virtual, with assessments and enrolment taking place in a patient’s home, made possible by the self-administration of medicines and end-to-end digital tools. This totally virtual model is gradually transitioning from early-phase and post-approval trials to massive pivotal trials. While most clinical trials will not be completely virtual, they will employ one or more Decentralized features based on their end targets, patient demographics, and treatments. Many of these ingredients have been extensively tested, with 48 to 95 percent of sponsors reporting use in at least one Phase III trial. Convenience is becoming increasingly important for patient enrolment and retention in clinical trials, particularly those involving uncommon diseases. Patients and physicians anticipate that sponsors would include patient convenience in trial designs, and investigators in many countries predict an increase in patient-centric trial characteristics. Electronic patient-reported outcomes (ePROs) and Electronic clinical outcomes assessments (eCOAs) make use of digital platforms to obtain direct data from research stakeholders. As a result, there is much less paper recording and much timelier data, as well as more patient consistency. When clinical research associates are unable to physically attend an investigator site or clinic, virtual solutions for generating data analytics, enabling remote source data verification, and performing other monitoring activities are available. Thus, the growing popularity of Decentralized clinical trials due its advantages is driving the market growth.
Discover sales predictions for the global decentralized clinical trials market and submarkets
Over the last few years, decentralized clinical trials has gained widespread attention due to its high efficiency and adoption by pharmaceutical & biopharmaceutical companies. Along with revenue prediction for the overall world market, there are 3 segmentations of the Decentralized Clinical Trials market, with forecasts for 3 Study Design, 5 Indication, 3 End-User, each forecasted at a global, regional, and country level, along with COVID-19 impact recovery pattern analysis for all segments.
Who are the leading players analysed in the market?
• CASTOR EDC
• Clinical Ink, Inc. (G. I. Partners)
• CLOUDZBY, INC.
• Dassault SystemesSE (Medidata Solutions, Inc.)
• ERGOMED PLC
• Florence Healthcare, Inc.
• ICON PLC
• IQVIA Holdings, Inc.
• Laboratory Corporation of America Holdings (Covance, Inc.)
• Medable, Inc.
• Medrio Inc.
• Oracle Corporation
• Parexel International Corporation
• Pharmaceutical Product Development, Inc.
• Syneos Health
To access the data contained in this document please email contactus@visiongain.com
Buy our report today Decentralized Clinical Trials Market Report 2022-2032: Forecasts by Study Design (Interventional Study Design, Observational Study Design, Expanded Access Study Design), by Indication (Oncology, Cardiovascular, Immunology, Respiratory, Others) by End-user (Pharmaceutical & Biopharmaceutical Companies, CROs, Others) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Recovery Scenarios. Avoid missing out by staying informed – order our report now.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for a specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: sara.peerun@visiongain.com
1.1 Introduction to Decentralised Clinical Trials Market
1.2 Why You Should Read This Report
1.3 What This Report Delivers
1.4 Key Questions Answered by This Analytical Report Include:
1.5 Who is This Report For?
1.6 Methodology
1.6.1 Primary Research
1.6.2 Secondary Research
1.6.3 Market Evaluation &Forecast Methodology
1.7 Frequently Asked Questions (FAQs)
1.8 Associated Visiongain Reports
1.9 About Visiongain
2 Executive Summary
3 Market Dynamics
3.1 Drivers for Decentralised Clinical Trials Market
3.1.1 Advantages of Decentralised Trials is driving the Market
3.1.2 Increasing R&D expenditure of pharmaceutical and biopharmaceutical companies
3.1.3 Growing Adoption of Advanced Technologies is Boosting the Market Growth
3.1.4 COVID-19 Pandemic is driving the Adoption of Decentralised Clinical Trials
3.1.5 Rising Presence of CROs in Emerging Market is Boosting the Market Growth
3.2 Restraints for Decentralised Clinical Trials Market
3.2.1 Data Security Concerns Hinders the Market Growth
3.2.2 Complex Supply Chain and Manufacturing Could limit the Entry of New Players
3.3 Opportunities for Decentralised Clinical Trials Market
3.3.1 Evolution of Decentralised Trials Technologies is Projected to Boost the Market Growth
3.3.2 Introduction of Advanced Cloud Platform
3.3.3 Support from Government is estimated to propel the Market Growth
3.3.4 Mergers, Agreements, and Acquisitions by the Market Players is a Major Trend Witnessed in the Market
3.4 Challenges for Decentralised Clinical Trials Market
3.4.1 Challenges associated With Clinical Trials Logistics could limit its Adoption
3.4.2 Lack of Global; Standardisation for Decentralised Clinical Trials
3.5 SWOT Analysis for Decentralised Clinical Trials Market
3.5.1 Strengths: Decentralised Clinical Trials Market
3.5.1.1 Patient-Centric Approach is a Major Strengths
3.5.2 Weaknesses: Decentralised Clinical Trials Market
3.5.2.1 Lack of Standard Regulations is a Major Issue
3.5.3 Opportunities: Decentralised Clinical Trials Market
3.5.3.1 Technological Advancements is projected to Strengthen the Market Growth in Near Future
3.5.4 Threats: Decentralised Clinical Trials Market
3.5.4.1 Data Security and Privacy Risk Associated with DCTs
3.6 Porter’s Five Forces Analysis: Decentralised Clinical Trials Market
3.6.1 Bargaining Power of Supplier: Low
3.6.2 Bargaining Power of Buyer: Moderate
3.6.3 Competitive Rivalry: High
3.6.4 Threat of New Entrants: Moderate
3.6.5 Threat of Substitutes: Moderate
3.7 PEST Analysis: Decentralised Clinical Trials Market
3.7.1 Political Factors Impacting Decentralised Clinical Trials Market
3.7.2 Economic Factors Impacting Decentralised Clinical Trials Market
3.7.3 Social Factors Impacting Decentralised Clinical Trials Market
3.7.4 Technological Factors Impacting Decentralised Clinical Trials Market
4 COVID-19 Impact: Global Decentralised Clinical Trials Market
4.1 Global Decentralised Clinical Trials Market Forecast, 2022-2032
4.2 COVID-19 Impact Overview
4.3 "V" shaped Recovery - Rapid Decline - Sharp Borrow -Rapid Recovery
4.4 "U" Shaped Recovery -Rapid Decline Early Then Slow - Gradual at the Bottom - Slow Recovery at First - Faster Recovery Later On
4.5 "W" Shaped Recovery -Rapid Decline - Rapid Recovery- Return of Virus - Another Sharp Decline -Recovery
4.6 "L" Shaped Recovery - Rapid Decline - Then Slow Growth
5 Global Decentralised Clinical Trials Market Size Forecast 2022-2032 by Study Design
5.1 Global Interventional Study Design Segment: Revenue Forecast 2022-2032
5.1.1 Interventional Study Design is the Largest Revenue Grossing Segment
5.1.2 Global Decentralised Clinical Trials Market for Interventional Study Design Segment Forecast, 2022-2032: Recovery Forecasts (V, U, W, L)
5.2 Global Observational Study Design Segment: Revenue Forecast 2022-2032
5.2.1 Observational Study Design
5.2.2 Global Decentralised Clinical Trials Market for Observational Study Design Segment Forecast, 2022-2032: Recovery Forecasts (V, U, W, L)
5.3 Global Expanded Access Study Design Segment: Revenue Forecast 2022-2032
5.3.1 Expanded Access Study Design is a Promising form of Adoptive Decentralised Clinical Trials
5.3.2 Global Decentralised Clinical Trials Market for Expanded Study Design Segment Forecast, 2022-2032: Recovery Forecasts (V, U, W, L)
7 Global Decentralised Clinical Trials Market Size Forecast 2022-2032 by Indication
7.1 Oncology Segment: Revenue Forecast 2022-2032
7.1.1 Decentralised Oncology Trial Operations Could Result in Significant Increases in Representation and Accessibility
7.1.2 Global Decentralised Clinical Trials Market for Oncology Segment Forecast, 2022-2032: Recovery Forecasts (V, U, W, L)
7.2 Cardiovascular Segment: Revenue Forecast 2022-2032
7.2.1 DCTs enhanced the Logistics of Clinical Trials for Cardiovascular Indications
7.2.2 Global Decentralised Clinical Trials Market for Cardiovascular Segment Forecast, 2022-2032: Recovery Forecasts (V, U, W, L)
7.3 Immunology Segment: Revenue Forecast 2022-2032
7.3.1 Removing Impediments to Site Visits Improves Immunological Disorder Trial Retention
7.3.2 Global Decentralised Clinical Trials Market for Immunology Segment Forecast, 2022-2032: Recovery Forecasts (V, U, W, L)
7.4 Respiratory Segment: Revenue Forecast 2022-2032
7.4.1 COVID-19 Pandemic has propelled the Use of DCT in Respiratory Diseases Clinical Trials
7.4.2 Global Decentralised Clinical Trials Market for Respiratory Segment Forecast, 2022-2032: Recovery Forecasts (V, U, W, L)
7.5 Others Segment: Revenue Forecast 2022-2032
7.5.1 Growing Demand for Advanced Drugs Is Expected to Propel the Others Segment Growth
7.5.2 Global Decentralised Clinical Trials Market for Others Segment Forecast, 2022-2032: Recovery Forecasts (V, U, W, L)
8 Decentralised Clinical Trials Market by End-User Forecast, 2022-2032
8.1 Pharmaceutical & Biopharmaceutical Companies Segment Market Forecast, 2022-2032
8.1.1 Largest-Revenue Growing Segment in the Market
8.1.2 Global Decentralised Clinical Trials Market for Pharmaceutical & Biopharmaceutical Companies Segment Forecast, 2022-2032: Recovery Scenarios (V, U, W, L)
8.2 CROs Segment Market Forecast, 2022-2032
8.2.1 Second-Largest Revenue Generating Segment
8.2.2 Global Decentralised Clinical Trials Market for CRO’s Segment Forecast, 2022-2032: Recovery Scenarios (V, U, W, L)
8.3 Others Segment Market Forecast, 2022-2032
8.3.1 Well-defined Regulations for the Cellular Therapies in the Developed Countries Is Expected to Drive the Market Growth
8.3.2 Global Decentralised Clinical Trials Market for Others Segment Forecast, 2022-2032: Recovery Scenarios (V, U, W, L)
9 Global Decentralised Clinical Trials Market Forecast, By Geography 2022-2032
9.1 Global Decentralised Clinical Trials Market by Region Forecast 2022-2032
9.1 Global Decentralised Clinical Trials Market for Region Segment Forecast, 2022-2032: Recovery Scenarios (V, U, W, L)
10 North America Decentralised Clinical Trials Market, 2022-2032
10.1 North America is the Largest Revenue Grossing Region in the Global Market
10.2 North America Decentralised Clinical Trials Market by Country, Forecast 2022-2032
10.3 North America Decentralised Clinical Trials Market Forecast by Study Design
10.3.1 Recovery Scenarios (V, U, W, L): North America Decentralised Clinical Trials Market Forecast by Study Type, 2022-2032
10.4 North America Decentralised Clinical Trials Market Forecast by Indication
10.4.1 Recovery Scenarios (V, U, W, L): North America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032
10.5 North America Decentralised Clinical Trials Market Forecast by End-User
10.5.1 Recovery Scenarios (V, U, W, L): North America Decentralised Clinical Trials Market Forecast by End-User, 2022-2032
10.6 U. S. Decentralised Clinical Trials Market Forecast, 2022-2032
10.6.1 Presence of Leading Players is a Major factor driving the U. S. Decentralised Clinical Trials Market
10.6.2 U. S. Decentralised Clinical Trials Market Growth is Primarily Attributable to Growing R&D Expenditure and Outsourcing Activities
10.6.3 Recovery Scenarios (V, U, W, L): U. S. Decentralised Clinical Trials Market Forecast, 2022-2032
10.7 Canada Decentralised Clinical Trials Market Forecast,2022-2032
10.7.1 Canada Market Anticipated to Witness the Fastest Growth in the North America Region
10.7.2 Growing Canadian research collaborations determined to Provide Infrastructure for Cell-Based Therapy Research
10.7.3 Recovery Scenarios (V, U, W, L): Canada Decentralised Clinical Trials Market Forecast, 2022-2032
11 Europe Decentralised Clinical Trials Market, 2022-2032
11.1 Second Largest Revenue Grossing Region in the Global Market
11.2 Europe Decentralised Clinical Trials Market by Country, Forecast 2022-2032
11.3 Europe Decentralised Clinical Trials Market Forecast by Study Design
11.3.1 Recovery Scenarios (V, U, W, L): Europe Decentralised Clinical Trials Market Forecast by Study Type, 2022-2032
11.4 Europe Decentralised Clinical Trials Market Forecast by Indication
11.4.1 Recovery Scenarios (V, U, W, L): Europe Decentralised Clinical Trials Market Forecast by Indication, 2022-2032
11.5 Europe Decentralised Clinical Trials Market Forecast by End-User
11.5.1 Recovery Scenarios (V, U, W, L): Europe Decentralised Clinical Trials Market Forecast by Indication, 2022-2032
11.6 Germany Decentralised Clinical Trials Market Forecast, 2022-2032
11.6.1 Germany Is Home to a Few of the Biggest Pharmaceutical Firms of the World that is Expected to Boost the Regional Market Growth
11.6.2 LINK Medical strengthens its position in Germany as a strategic gateway between Europe and the Nordics
11.6.3 Recovery Scenarios (V, U, W, L): Germany Decentralised Clinical Trials Market Forecast, 2022-2032
11.7 UK Decentralised Clinical Trials Market Forecast, 2022-2032
11.7.1 Presence of Well-Developed R&D Infrastructure
11.7.2 UK has been a Hub for Generics and Biosimilar Drugs Production and Development
11.7.3 Recovery Scenarios (V, U, W, L): UK Decentralised Clinical Trials Market Forecast, 2022-2032
11.8 France Decentralised Clinical Trials Market Forecast, 2022-2032
11.8.1 Rising Preference for France for Drug R&D and Manufacturing by Leading Players
11.8.2 Major Player focusing on Collaboration and product development strategies in this region
11.8.3 Recovery Scenarios (V, U, W, L): France Decentralised Clinical Trials Market Forecast, 2022-2032
11.9 Italy Decentralised Clinical Trials Market Forecast, 2022-2032
11.9.1 Pharmaceutical Manufacturing Hub
11.9.2 Italian Decentralised Clinical Trials Market Is the Fourth Biggest Market Within the European Region
11.9.3 Recovery Scenarios (V, U, W, L): Italy Decentralised Clinical Trials Market Forecast, 2022-2032
11.10 Spain Decentralised Clinical Trials Market Forecast, 2022-2032
11.10.1 Demand for Drugs Is Influenced by the Growth of the Pharmaceutical Industry
11.10.2 Spanish Economy Is Growing Strong
11.10.3 Recovery Scenarios (V, U, W, L): Spain Decentralised Clinical Trials Market Forecast, 2022-2032
11.11 Russia Decentralised Clinical Trials Market Forecast, 2022-2032
11.11.1 Unmet Pharmaceutical Needs
11.11.2 Russia’s attractiveness as a location for all types of clinical trial
11.11.3 Recovery Scenarios (V, U, W, L): Russia Decentralised Clinical Trials Market Forecast, 2022-2032
11.12 Rest of Europe Decentralised Clinical Trials Market Forecast, 2022-2032
11.12.1 Increasing Prevalence of Chronic Diseases Drives the Market Growth in this Region
11.12.2 Recovery Scenarios (V, U, W, L): Rest of Europe Decentralised Clinical Trials Market Forecast, 2022-2032
12 Asia Pacific Decentralised Clinical Trials Market, 2022-2032
12.1 Asia Pacific is the Fastest Growing Market
12.2 Asia Pacific Decentralised Clinical Trials Market by Country, Forecast 2022-2032
12.3 Asia Pacific Decentralised Clinical Trials Market Forecast by Study Design
12.3.1 Recovery Scenarios (V, U, W, L): Asia Pacific Decentralised Clinical Trials Market Forecast by Study Type, 2022-2032
12.4 Asia Pacific Decentralised Clinical Trials Market Forecast by Indication
12.4.1 Recovery Scenarios (V, U, W, L): Asia Pacific Decentralised Clinical Trials Market Forecast by Indication, 2022-2032
12.5 Asia Pacific Decentralised Clinical Trials Market Forecast by End-User
12.5.1 Recovery Scenarios (V, U, W, L): Asia Pacific Decentralised Clinical Trials Market Forecast by Indication, 2022-2032
12.6 Japan Decentralised Clinical Trials Market Forecast, 2022-2032
12.6.1 Japan Accounted for the Largest Market Share in the Asia Pacific Region
12.6.2 International CROs that Have Established Branches in Japan has propelled the Market Growth
12.6.3 Recovery Scenarios (V, U, W, L): Japan Decentralised Clinical Trials Market Forecast, 2022-2032
12.7 China Decentralised Clinical Trials Market Forecast, 2022-2032
12.7.1 Strategic Initiatives Undertaken by the Market Players
12.7.2 Increasing Healthcare Spending in China
12.7.3 Recovery Scenarios (V, U, W, L): China Decentralised Clinical Trials Market Forecast, 2022-2032
12.8 India Decentralised Clinical Trials Market Forecast, 2022-2032
12.8.1 Developing Healthcare Infrastructure
12.8.2 India Poised to conduct more Decentralised Clinical Trials
12.8.3 Recovery Scenarios (V, U, W, L): India Decentralised Clinical Trials Market Forecast, 2022-2032
12.9 Australia Decentralised Clinical Trials Market Forecast, 2022-2032
12.9.1 Australia Has as Long a History of Conducting High-Standard Clinical Trials
12.9.2 Availability Of High-End Healthcare Infrastructure
12.9.3 Recovery Scenarios (V, U, W, L): Australia Decentralised Clinical Trials Market Forecast, 2022-2032
12.10 South Korea Decentralised Clinical Trials Market Forecast, 2022-2032
12.10.1 Adoption of Advanced Solutions by Hospitals & CROs among People
12.10.2 Availability of Skilled Workforce is expected to drive the South Korea Market
12.10.3 Recovery Scenarios (V, U, W, L): South Korea Decentralised Clinical Trials Market Forecast, 2022-2032
12.11 Rest of Asia Pacific Decentralised Clinical Trials Market Forecast, 2022-2032
12.11.1 Rising Disposable Income
12.11.2 Recovery Scenarios (V, U, W, L): Rest of Asia Pacific Decentralised Clinical Trials Market Forecast, 2022-2032
13 Latin America Decentralised Clinical Trials Market, 2022-2032
13.1 Latin America Decentralised Clinical Trials Market
13.2 Latin America Decentralised Clinical Trials Market by Country, Forecast 2022-2032
13.3 Latin America Decentralised Clinical Trials Market Forecast by Study Design
13.3.1 Recovery Scenarios (V, U, W, L): Latin America Decentralised Clinical Trials Market Forecast by Study Type, 2022-2032
13.4 Latin America Decentralised Clinical Trials Market Forecast by Indication
13.4.1 Recovery Scenarios (V, U, W, L): Latin America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032
13.5 Latin America Decentralised Clinical Trials Market Forecast by End-User
13.5.1 Recovery Scenarios (V, U, W, L): Latin America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032
13.6 Brazil Decentralised Clinical Trials Market Forecast, 2022-2032
13.6.1 Fastest Growing Economy in Latin America
13.6.2 High Potential Manufacturing Environment
13.6.3 Recovery Scenarios (V, U, W, L): Brazil Decentralised Clinical Trials Market Forecast, 2022-2032
13.7 Mexico Decentralised Clinical Trials Market Forecast, 2022-2032
13.7.1 Lower Manufacturing Costs than In the U. S.
13.7.2 Strategic Initiatives Undertaken by Market Players
13.7.3 Recovery Scenarios (V, U, W, L): Mexico Decentralised Clinical Trials Market Forecast, 2022-2032
13.8 Rest of Latin America Decentralised Clinical Trials Market Forecast, 2022-2032
13.8.1 Evolving Healthcare Infrastructure in Latin America
13.8.2 Recovery Scenarios (V, U, W, L): Rest of Latin America Decentralised Clinical Trials Market Forecast, 2022-2032
14 MEA Decentralised Clinical Trials Market, 2022-2032
14.1 Rising Prevalence of Cancer in MEA Region
14.2 MEA Decentralised Clinical Trials Market by Country, Forecast 2022-2032
14.3 MEA Decentralised Clinical Trials Market Forecast by Study Design
14.3.1 Recovery Scenarios (V, U, W, L): MEA Decentralised Clinical Trials Market Forecast by Study Type, 2022-2032
14.4 MEA Decentralised Clinical Trials Market Forecast by Indication
14.4.1 Recovery Scenarios (V, U, W, L): MEA Decentralised Clinical Trials Market Forecast by Indication, 2022-2032
14.5 MEA Decentralised Clinical Trials Market Forecast by End-User
14.5.1 Recovery Scenarios (V, U, W, L): MEA Decentralised Clinical Trials Market Forecast by Indication, 2022-2032
14.6 South Africa Decentralised Clinical Trials Market Forecast, 2022-2032
14.6.1 Prevalence of Cancer in South Africa is Increasing Rapidly
14.6.2 Population Exposed to Mining Suffers from Cancer
14.6.3 Recovery Scenarios (V, U, W, L): South Africa Decentralised Clinical Trials Market Forecast, 2022-2032
14.7 GCC Decentralised Clinical Trials Market Forecast, 2022-2032
14.7.1 Presence of Huge Growth Opportunities
14.7.2 Advances in personalised medicine and immuno-oncology have ushered in a paradigm change
14.7.3 Recovery Scenarios (V, U, W, L): GCC Decentralised Clinical Trials Market Forecast, 2022-2032
14.8 Rest of MEA Decentralised Clinical Trials Market Forecast, 2022-2032
14.8.1 MEA DCT market is positioned as a new ally for the worldwide
14.8.2 Extensive Tobacco Use Increases Incidence of Cancer
14.8.3 Recovery Scenarios (V, U, W, L): Rest of MEA Decentralised Clinical Trials Market Forecast, 2022-2032
15 Company Profiles: Decentralised Clinical Trials Market
15.1 Decentralised Clinical Trials Market: Company Share Analysis
15.2 Decentralised Clinical Trials Market Recent Developments, 2018-2021
15.3 IQVIA Holdings, Inc.
15.3.1 Company Snapshot
15.3.2 Company Overview
15.3.3 Financial Analysis
15.3.3.1 Net Revenue, 2016-2020
15.3.3.2 Regional Revenue/Market Shares, 2020
15.3.3.3 Segmental Revenue/Market Shares, 2020
15.3.4 Product Benchmarking
15.3.5 Recent Developments, 2018-2021
15.4 ICON PLC
15.4.1 Company Snapshot
15.4.2 Company Overview
15.4.3 Financial Analysis
15.4.3.1 Net Revenue, 2016-2020
15.4.3.2 Regional Revenue/Market Shares, 2020
15.4.4 Product Benchmarking
15.4.5 Recent Developments, 2018-2021
15.5 Laboratory Corporation of America Holdings (Covance, Inc. )
15.5.1 Company Snapshot
15.5.2 Company Overview
15.5.3 Financial Analysis
15.5.3.1 Net Revenue, 2016-2020
15.5.3.2 Regional Revenue/Market Shares, 2020
15.5.3.3 Segmental Revenue/Market Shares, 2020
15.5.4 Product Benchmarking
15.5.5 Recent Developments, 2018-2021
15.6 Dassault SystemesSE (Medidata Solutions, Inc. )
15.6.1 Company Snapshot
15.6.2 Company Overview
15.6.3 Financial Analysis
15.6.3.1 Net Revenue, 2016-2020
15.6.3.2 Regional Revenue/Market Shares, 2020
15.6.3.3 Segmental Revenue/Market Shares, 2020
15.6.4 Product Benchmarking
15.6.5 Recent Developments, 2018-2021
15.7 Oracle Corporation
15.7.1 Company Snapshot
15.7.2 Company Overview
15.7.3 Financial Analysis
15.7.3.1 Net Revenue, 2016-2020
15.7.3.2 Regional Revenue/Market Shares, 2020
15.7.3.3 Segmental Revenue/Market Shares, 2020
15.7.4 Product Benchmarking
15.7.5 Recent Developments, 2018-2021
15.8 Parexel International Corporation (Pamplona Capital Management)
15.8.1 Company Snapshot
15.8.2 Company Overview
15.8.3 Product Benchmarking
15.8.4 Recent Developments, 2018-2021
15.9 Medrio Inc.
15.9.1 Company Snapshot
15.9.2 Company Overview
15.9.3 Product Benchmarking
15.9.4 Recent Developments, 2018-2021
15.10 Medable, Inc.
15.10.1 Company Snapshot
15.10.2 Company Overview
15.10.3 Product Benchmarking
15.10.4 Recent Developments, 2018-2021
15.11 Clinical Ink, Inc. (G. I. Partners)
15.11.1 Company Snapshot
15.11.2 Company Overview
15.11.3 Product Benchmarking
15.11.4 Recent Developments, 2018-2021
15.12 FLORENCE HEALTHCARE INC.
15.12.1 Company Snapshot
15.12.2 Company Overview
15.12.3 Product Benchmarking
15.12.4 Recent Developments, 2018-2021
15.13 Syneos Health
15.13.1 Company Snapshot
15.13.2 Company Overview
15.13.3 Financial Analysis
15.13.3.1 Net Revenue, 2016-2020
15.13.3.2 Regional Revenue/Market Shares, 2020
15.13.3.3 Segmental Revenue/Market Shares, 2020
15.13.4 Product Benchmarking
15.13.5 Recent Developments, 2018-2021
15.14 Pharmaceutical Product Development, Inc.
15.14.1 Company Snapshot
15.14.2 Company Overview
15.14.3 Financial Analysis
15.14.3.1 Net Revenue, 2016-2020
15.14.3.2 Regional Revenue/Market Shares, 2020
15.14.3.3 Segmental Revenue/Market Shares, 2020
15.14.4 Product Benchmarking
15.14.5 Recent Developments, 2018-2021
15.15 CASTOR EDC
15.15.1 Company Snapshot
15.15.2 Company Overview
15.15.3 Product Benchmarking
15.15.4 Recent Developments, 2018-2021
15.16 CLOUDZBY, INC.
15.16.1 Company Snapshot
15.16.2 Company Overview
15.16.3 Product Benchmarking
15.16.4 Recent Developments, 2018-2021
15.17 ERGOMED PLC
15.17.1 Company Snapshot
15.17.2 Company Overview
15.17.3 Financial Analysis
15.17.3.1 Net Revenue, 2016-2020
15.17.3.2 Regional Revenue/Market Shares, 2020
15.17.3.3 Segmental Revenue/Market Shares, 2020
15.17.4 Product Benchmarking
15.17.5 Recent Developments, 2018-2021
16 Conclusion and Recommendations
16.1 Concluding Remarks from Visiongain
16.2 Recommendations for Market Players
16.3 Leading Regions and Fastest Growing Region (North America Region Accounted for the Largest Market Share)
List of Tables
Table 1 Global Decentralized Clinical Trials Market, 2022 & 2032 (US$ mn, CAGR %)
Table 2 R&D expenditure of Major Pharmaceutical and Biopharmaceutical Companies
Table 3 Global Decentralized Clinical Trials Market Size Forecast: Revenue ($mn), AGR (%), CAGR (%), 2022-2032
Table 4 Global Decentralized Clinical Trials Market Size Forecast: Revenue ($mn), AGR (%), CAGR (%), 2022-2032: "V" Shaped Recovery
Table 5 Global Decentralized Clinical Trials Market Size Forecast: Revenue ($mn), AGR (%), CAGR (%), 2022-2032: "U" Shaped Recovery
Table 6 Global Decentralized Clinical Trials Market Size Forecast: Revenue ($mn), AGR (%), CAGR (%), 2022-2032: "W" Shaped Recovery
Table 7 Global Decentralized Clinical Trials Market Size Forecast: Revenue ($mn), AGR (%), CAGR (%), 2022-2032: "L" Shaped Recovery
Table 8 Global Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million, AGR %, CAGR %)
Table 9 Global Interventional Study Design Segment: Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 10 “V” Shaped Recovery: Global Interventional Study Design Segment: Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 11 “U” Shaped Recovery: Global Interventional Study Design Segment: Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 12 “W” Shaped Recovery: Global Interventional Study Design Segment: Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 13 “L” Shaped Recovery: Global Interventional Study Design Segment: Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 14 Global Observational Study Design Segment: Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 15 “V” Shaped Recovery: Global Observational Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 16 “U” Shaped Recovery: Global Observational Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 17 “W” Shaped Recovery: Global Observational Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 18 “L” Shaped Recovery: Global Observational Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 19 Global Expanded Access Study Design Segment: Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 20 “V” Shaped Recovery: Global Expanded Access Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 21 “U” Shaped Recovery: Global Expanded Access Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 22 “W” Shaped Recovery: Global Expanded Access Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 23 “L” Shaped Recovery: Global Expanded Access Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 24 Global Decentralized Clinical Trials Market Size Forecast by Indication, 2022-2032 (US$ million, AGR %, CAGR %)
Table 25 Global Oncology Segment: Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 26 “V” Shaped Recovery: Global Oncology Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 27 “U” Shaped Recovery: Global Oncology Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 28 “W” Shaped Recovery: Global Oncology Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 29 “L” Shaped Recovery: Global Oncology Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 30 Global Cardiovascular Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 31 “V” Shaped Recovery: Global oncology Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 32 “U” Shaped Recovery: Global Cardiovascular Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 33 “W” Shaped Recovery: Global Cardiovascular Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 34 “L” Shaped Recovery: Global Cardiovascular Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 35 Global Immunology Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 36 “V” Shaped Recovery: Global Immunology Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 37 “U” Shaped Recovery: Global Immunology Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 38 “W” Shaped Recovery: Global Immunology Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 39 “L” Shaped Recovery: Global Immunology Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 40 Global Respiratory Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 41 “V” Shaped Recovery: Global Respiratory Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 42 “U” Shaped Recovery: Global Respiratory Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 43 “W” Shaped Recovery: Global Respiratory Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 44 “L” Shaped Recovery: Global Respiratory Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 45 Global Others Segment: Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 46 “V” Shaped Recovery: Global Others Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 47 “U” Shaped Recovery: Global Others Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 48 “W” Shaped Recovery: Global Others Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 49 “L” Shaped Recovery: Global Others Segment, Revenue Forecast 2022-2032 (US$ million, AGR%, CAGR %)
Table 50 Global Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 51 Global Decentralized Clinical Trials Market for Pharmaceutical & Biopharmaceutical Companies Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 52 Global Decentralized Clinical Trials Market for Pharmaceutical & Biopharmaceutical Companies Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 53 Global Decentralized Clinical Trials Market for Pharmaceutical & Biopharmaceutical Companies Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 54 Global Decentralized Clinical Trials Market for Pharmaceutical & Biopharmaceutical Companies Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 55 Global Decentralized Clinical Trials Market for Pharmaceutical & Biopharmaceutical Companies Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 56 Global Decentralized Clinical Trials Market for CROs Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 57 Global Decentralized Clinical Trials Market for CROs Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 58 Global Decentralized Clinical Trials Market for CROs Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 59 Global Decentralized Clinical Trials Market for CROs Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 60 Global Decentralized Clinical Trials Market for CROs Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 61 Global Decentralized Clinical Trials Market for Others Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 62 Global Decentralized Clinical Trials Market for Others Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 63 Global Decentralized Clinical Trials Market for Others Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 64 Global Decentralized Clinical Trials Market for Others Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 65 Global Decentralized Clinical Trials Market for Others Segment Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 66 Global Decentralized Clinical Trials Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 67 Global Decentralized Clinical Trials Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 68 Global Decentralized Clinical Trials Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 69 Global Decentralized Clinical Trials Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 70 Global Decentralized Clinical Trials Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 71 North America Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 72 North America Decentralized Clinical Trials Market Forecast by Country, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 73 North America Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million, AGR %, CAGR %)
Table 74 North America Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "V" Shaped Recovery
Table 75 North America Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "U" Shaped Recovery
Table 76 North America Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "W" Shaped Recovery
Table 77 North America Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "L" Shaped Recovery
Table 78 North America Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 79 North America Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 80 North America Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 81 North America Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 82 North America Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 83 North America Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 84 North America Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 85 North America Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 86 North America Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 87 North America Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 88 U. S. Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 89 U. S. Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 90 U. S. Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 91 U. S. Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 92 U. S. Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 93 Canada Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 94 Canada Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 95 Canada Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 96 Canada Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 97 Canada Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 98 Europe Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 99 Europe Decentralized Clinical Trials Market Forecast by Country, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 100 Europe Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %)
Table 101 Europe Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "V" Shaped Recovery
Table 102 Europe Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "U" Shaped Recovery
Table 103 Europe Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "W" Shaped Recovery
Table 104 Europe Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "L" Shaped Recovery
Table 105 Europe Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 106 Europe Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 107 Europe Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 108 Europe Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 109 Europe Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 110 Europe Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 111 Europe Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 112 Europe Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 113 Europe Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 114 Europe Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 115 Germany Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 116 Germany Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 117 Germany Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 118 Germany Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 119 Germany Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 120 UK Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 121 UK Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 122 UK Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 123 UK Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 124 UK Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 125 France Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 126 France Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 127 France Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 128 France Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 129 France Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 130 Italy Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 131 Italy Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 132 Italy Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 133 Italy Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 134 Italy Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 135 Spain Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 136 Spain Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 137 Spain Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 138 Spain Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 139 Spain Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 140 Russia Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 141 Russia Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 142 Russia Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 143 Russia Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 144 Russia Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 145 Rest of Europe Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 146 Rest of Europe Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 147 Rest of Europe Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 148 Rest of Europe Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 149 Rest of Europe Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 150 Asia Pacific Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 151 Asia Pacific Decentralized Clinical Trials Market Forecast by Country, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 152 Asia Pacific Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %)
Table 153 Asia Pacific Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "V" Shaped Recovery
Table 154 Asia Pacific Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "U" Shaped Recovery
Table 155 Asia Pacific Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "W" Shaped Recovery
Table 156 Asia Pacific Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "L" Shaped Recovery
Table 157 Asia Pacific Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 158 Asia Pacific Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 159 Asia Pacific Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 160 Asia Pacific Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 161 Asia Pacific Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 162 Asia Pacific Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 163 Asia Pacific Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 164 Asia Pacific Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 165 Asia Pacific Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 166 Asia Pacific Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 167 Japan Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 168 Japan Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 169 Japan Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 170 Japan Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 171 Japan Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 172 China Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 173 China Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 174 China Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 175 China Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 176 China Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 177 India Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 178 India Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 179 India Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 180 India Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 181 India Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 182 Australia Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 183 Australia Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 184 Australia Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 185 Australia Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 186 Australia Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 187 South Korea Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 188 South Korea Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 189 South Korea Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 190 South Korea Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 191 South Korea Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 192 Rest of Asia Pacific Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 193 Rest of Asia Pacific Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 194 Rest of Asia Pacific Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 195 Rest of Asia Pacific Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 196 Rest of Asia Pacific Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 197 Latin America Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 198 Latin America Decentralized Clinical Trials Market Forecast by Country, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 199 Latin America Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %)
Table 200 Latin America Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "V" Shaped Recovery
Table 201 Latin America Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "U" Shaped Recovery
Table 202 Latin America Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "W" Shaped Recovery
Table 203 Latin America Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "L" Shaped Recovery
Table 204 Latin America Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 205 Latin America Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 206 Latin America Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 207 Latin America Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 208 Latin America Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 209 Latin America Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 210 Latin America Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 211 Latin America Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 212 Latin America Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 213 Latin America Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 214 Brazil Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 215 Brazil Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 216 Brazil Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 217 Brazil Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 218 Brazil Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 219 Mexico Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 220 Mexico Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 221 Mexico Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 222 Mexico Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 223 Mexico Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 224 Rest of Latin America Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 225 Rest of Latin America Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 226 Rest of Latin America Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 227 Rest of Latin America Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 228 Rest of Latin America Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 229 MEA Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 230 MEA Decentralized Clinical Trials Market Forecast by Country, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 231 MEA Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %)
Table 232 MEA Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "V" Shaped Recovery
Table 233 MEA Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "U" Shaped Recovery
Table 234 MEA Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "W" Shaped Recovery
Table 235 MEA Decentralized Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ mn, AGR %, CAGR %): "L" Shaped Recovery
Table 236 MEA Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 237 MEA Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 238 MEA Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 239 MEA Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 240 MEA Decentralized Clinical Trials Market Forecast by Indication, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 241 MEA Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 242 MEA Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 243 MEA Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 244 MEA Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 245 MEA Decentralized Clinical Trials Market Forecast by End-User, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 246 South Africa Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 247 South Africa Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Table 248 South Africa Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 249 South Africa Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 250 South Africa Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 251 South Africa Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 252 GCC Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 253 GCC Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 254 GCC Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 255 GCC Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 256 GCC Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 257 Rest of MEA Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR %)
Table 258 Rest of MEA Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
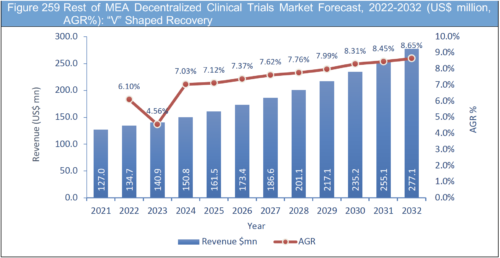
Table 259 Rest of MEA Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 260 Rest of MEA Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 261 Rest of MEA Decentralized Clinical Trials Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 262 Decentralized Clinical Trials Market Recent Developments, 2018-2021
Table 263 IQVIA Holdings, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 264 IQVIA Holdings, Inc.: Product Benchmarking
Table 265 Recent Developments, 2018-2021
Table 266 IQVIA Holdings, Inc.: Recent Developments, 2018-2021
Table 267 ICON PLC: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 268 ICON PLC: Product Benchmarking
Table 269 ICON PLC: Recent Developments, 2018-2021
Table 270 Laboratory Corporation of America Holdings: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 271 Laboratory Corporation of America Holdings: Product Benchmarking
Table 272 Laboratory Corporation of America Holdings: Recent Developments, 2018-2021
Table 273 Dassault SystemesSE: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 274 Dassault Systemes SE: Product Benchmarking
Table 275 Dassault Systemes SE: Recent Developments, 2018-2021
Table 276 Oracle Corporation: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 277 Oracle Corporation: Product Benchmarking
Table 278 Oracle Corporation: Recent Developments, 2018-2021
Table 279 Paraxel International Corporation: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 280 Paraxel International Corporation: Product Benchmarking
Table 281 Medrio Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 282 Medrio Inc.: Product Benchmarking
Table 283 Medrio Inc.: Recent Developments, 2018-2021
Table 284 Medable, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 285 Medable Inc.: Product Benchmarking
Table 286 Medable Inc.: Recent Developments, 2018-2021
Table 287 Clinical Ink, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 288 Clinical Ink, Inc.: Product Benchmarking
Table 289 Clinical Ink, Inc.: Recent Developments, 2018-2021
Table 290 Florence Healthcare, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 291 Florence Healthcare, Inc.: Product Benchmarking
Table 292 Florence Healthcare, Inc.: Recent Developments, 2018-2021
Table 293 Syneos Health: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 294 Syneos Health: Product Benchmarking
Table 1 Syneos Health: Recent Developments, 2018-2021
Table 295 PPD, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 296 PPD Inc.: Product Benchmarking
Table 297 PPD Inc.: Recent Developments, 2018-2021
Table 298 CASTOR EDC: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 299 CASTOR EDC: Product Benchmarking
Table 300 CASTOR EDC: Recent Developments, 2018-2021
Table 301 CLOUDZBY, INC.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 302 CLOUDZBY, INC.: Product Benchmarking
Table 303 CLOUDZBY, INC.: Recent Developments, 2018-2021
Table 304 Ergomed PLC: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 305 Ergomed PLC: Product Benchmarking
Table 306 Ergomed PLC: Recent Developments, 2018-2021
List of Figures
Figure 1 Global Decentralised Clinical Trials Market Size Forecast 2022-2032 (US$mn, AGR %)
Figure 2 Global Decentralised Clinical Trials Market Segmentation
Figure 3 Global Decentralised Clinical Trials Market: Market Trends
Figure 4 Global Decentralised Clinical Trials Market: SWOT Analysis
Figure 5 Global Decentralised Clinical Trials Market: Porter’s Five Forces Analysis
Figure 6 Global Decentralised Clinical Trials Market: PEST Analysis
Figure 7 Global Decentralised Clinical Trials Market Size Forecast 2022-2032 (US$mn, AGR %)
Figure 8 Global Decentralised Clinical Trials Market Size Forecast 2022-2032 (US$mn, AGR %): "V" Shaped Recovery
Figure 9 Global Decentralised Clinical Trials Market Size Forecast 2022-2032 (US$mn, AGR %): "U" Shaped Recovery
Figure 10 Global Decentralised Clinical Trials Market Size Forecast 2022-2032 (US$mn, AGR %): "W" Shaped Recovery
Figure 11 Global Decentralised Clinical Trials Market Size Forecast 2022-2032 (US$mn, AGR %): "L" Shaped Recovery
Figure 12 Global Interventional Study Design Segment: Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 13 “V” Shaped Recovery: Global Interventional Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 14 “U” Shaped Recovery: Global Interventional Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR%)
Figure 15 “W” Shaped Recovery: Global Interventional Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 16 “L” Shaped Recovery: Global Interventional Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 17 Global Observational Study Design Segment: Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 18 “V” Shaped Recovery: Global Observational Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 19 “U” Shaped Recovery: Global Observational Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 20 “W” Shaped Recovery: Global Observational Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR%)
Figure 21 “L” Shaped Recovery: Global Observational Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 22 Global Expanded Access Study Design Segment: Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 23 “V” Shaped Recovery: Global Expanded Access Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 24 “U” Shaped Recovery: Global Expanded Access Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 25 “W” Shaped Recovery: Global Expanded Access Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 26 “L” Shaped Recovery: Global Expanded Access Study Design Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 27 Global Decentralised Clinical Trials Market Share Forecast by Indication, 2022 2027 2032 (%)
Figure 28 Global Oncology Segment: Revenue Forecast 2022-2032 (US$ million, AGR%)
Figure 29 “V” Shaped Recovery: Global Oncology Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 30 “U” Shaped Recovery: Global Oncology Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 31 “W” Shaped Recovery: Global Oncology Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 32 “L” Shaped Recovery: Global Oncology Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 33 Global Cardiovascular Segment: Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 34 “V” Shaped Recovery: Global Cardiovascular Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 35 “U” Shaped Recovery: Global Cardiovascular Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 36 “W” Shaped Recovery: Global Cardiovascular Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 37 “L” Shaped Recovery: Global Cardiovascular Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 38 Global Immunology Segment: Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 39 “V” Shaped Recovery: Global Immunology Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 40 “U” Shaped Recovery: Global Immunology Segment, Revenue Forecast 2022-2032 (US$ million, AGR%)
Figure 41 “W” Shaped Recovery: Global Immunology Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 42 “L” Shaped Recovery: Global Immunology Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 43 Global Respiratory Segment: Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 44 “V” Shaped Recovery: Global Respiratory Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 45 “U” Shaped Recovery: Global Respiratory Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 46 “W” Shaped Recovery: Global Respiratory Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 47 “L” Shaped Recovery: Global Respiratory Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 48 Global Others Segment: Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 49 “V” Shaped Recovery: Global Others Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 50 “U” Shaped Recovery: Global Others Segment, Revenue Forecast 2022-2032 (US$ million, AGR%)
Figure 51 “W” Shaped Recovery: Global Others Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 52 “L” Shaped Recovery: Global Others Segment, Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 53 Global Decentralised Clinical Trials Market Share Forecast by End-User, 2022 2027 2032 (%)
Figure 54 Global Decentralised Clinical Trials Market for Pharmaceutical & Biopharmaceutical Companies Segment: Revenue Forecast 2022-2032 (US$ million, AGR%)
Figure 55 Global Decentralised Clinical Trials Market for Pharmaceutical & Biopharmaceutical Companies Segment, Revenue Forecast 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 56 Global Decentralised Clinical Trials Market for Pharmaceutical & Biopharmaceutical Companies Segment, Revenue Forecast 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 57 Global Decentralised Clinical Trials Market for Pharmaceutical & Biopharmaceutical Companies Segment, Revenue Forecast 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 58 Global Decentralised Clinical Trials Market for Pharmaceutical & Biopharmaceutical Companies Segment, Revenue Forecast 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 59 Global Decentralised Clinical Trials Market for CROs Segment: Revenue Forecast 2022-2032 (US$ million, AGR%)
Figure 60 Global Decentralised Clinical Trials Market for CROs Segment, Revenue Forecast 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 61 Global Decentralised Clinical Trials Market for CROs Segment, Revenue Forecast 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 62 Global Decentralised Clinical Trials Market for CROs Segment, Revenue Forecast 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 63 Global Decentralised Clinical Trials Market for CROs Segment, Revenue Forecast 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 64 Global Decentralised Clinical Trials Market for Others Segment: Revenue Forecast 2022-2032 (US$ million, AGR %)
Figure 65 Global Decentralised Clinical Trials Market for Others Segment, Revenue Forecast 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 66 Global Decentralised Clinical Trials Market for Others Segment, Revenue Forecast 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 67 Global Decentralised Clinical Trials Market for Others Segment, Revenue Forecast 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 68 Global Decentralised Clinical Trials Market for Others Segment, Revenue Forecast 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 69 Global Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million)
Figure 70 Global Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million): “V” Shaped Recovery
Figure 71 Global Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million): “U” Shaped Recovery
Figure 72 Global Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 73 Global Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 74 North America Decentralised Clinical Trials Market Forecast 2022-2032 (US$ million, AGR %)
Figure 75 North America Decentralised Clinical Trials Market Forecast by Country 2022-2032 (US$ million)
Figure 76 North America Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million)
Figure 77 North America Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 78 North America Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 79 North America Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 80 North America Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 81 North America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million)
Figure 82 North America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 83 North America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 84 North America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 85 North America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 86 North America Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million)
Figure 87 North America Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 88 North America Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 89 North America Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 90 North America Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 91 U. S. Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 92 U. S. Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 93 U. S. Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 94 U. S. Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 95 U. S. Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 96 Canada Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 97 Canada Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 98 Canada Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 99 Canada Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 100 Canada Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 101 Europe Decentralised Clinical Trials Market Forecast 2022-2032 (US$ million, AGR%)
Figure 102 Europe Decentralised Clinical Trials Market Forecast by Country 2022-2032 (US$ million)
Figure 103 Europe Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million)
Figure 104 Europe Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 105 Europe Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 106 Europe Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 107 Europe Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 108 Europe Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million)
Figure 109 Europe Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 110 Europe Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 111 Europe Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 112 Europe Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 113 Europe Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million)
Figure 114 Europe Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 115 Europe Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 116 Europe Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 117 Europe Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 118 Germany Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 119 Germany Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 120 Germany Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 121 Germany Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 122 Germany Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 123 UK Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 124 UK Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 125 UK Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 126 UK Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 127 UK Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 128 France Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 129 France Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 130 France Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 131 France Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 132 France Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 133 Italy Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 134 Italy Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 135 Italy Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 136 Italy Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 137 Italy Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 138 Spain Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 139 Spain Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 140 Spain Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 141 Spain Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 142 Spain Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 143 Russia Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 144 Russia Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 145 Russia Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 146 Russia Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 147 Russia Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 148 Rest of Europe Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 149 Rest of Europe Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 150 Rest of Europe Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 151 Rest of Europe Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 152 Rest of Europe Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 153 Asia Pacific Decentralised Clinical Trials Market Forecast 2022-2032 (US$ million, AGR %)
Figure 154 Asia Pacific Decentralised Clinical Trials Market Forecast by Country 2022-2032 (US$ million)
Figure 155 Asia Pacific Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million)
Figure 156 Asia Pacific Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 157 Asia Pacific Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 158 Asia Pacific Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 159 Asia Pacific Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 160 Asia Pacific Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million)
Figure 161 Asia Pacific Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 162 Asia Pacific Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 163 Asia Pacific Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 164 Asia Pacific Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 165 Asia Pacific Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million)
Figure 166 Asia Pacific Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 167 Asia Pacific Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 168 Asia Pacific Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 169 Asia Pacific Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 170 Japan Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 171 Japan Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 172 Japan Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 173 Japan Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 174 Japan Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 175 China Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 176 China Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 177 China Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 178 China Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 179 China Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 180 India Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 181 India Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 182 India Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 183 India Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 184 India Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 185 Australia Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 186 Australia Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 187 Australia Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 188 Australia Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 189 Australia Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 190 South Korea Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 191 South Korea Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 192 South Korea Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 193 South Korea Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 194 South Korea Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 195 Rest of Asia Pacific Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 196 Rest of Asia Pacific Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 197 Rest of Asia Pacific Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 198 Rest of Asia Pacific Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 199 Rest of Asia Pacific Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 200 Latin America Decentralised Clinical Trials Market Forecast 2022-2032 (US$ million, AGR%)
Figure 201 Latin America Decentralised Clinical Trials Market Forecast by Country 2022-2032 (US$ million)
Figure 202 Latin America Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million)
Figure 203 Latin America Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 204 Latin America Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 205 Latin America Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 206 Latin America Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 207 Latin America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million)
Figure 208 Latin America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 209 Latin America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 210 Latin America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 211 Latin America Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 212 Latin America Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million)
Figure 213 Latin America Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 214 Latin America Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 215 Latin America Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 216 Latin America Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 217 Brazil Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 218 Brazil Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 219 Brazil Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 220 Brazil Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 221 Brazil Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 222 Mexico Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 223 Mexico Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 224 Mexico Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 225 Mexico Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 226 Mexico Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 227 Rest of Latin America Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 228 Rest of Latin America Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 229 Rest of Latin America Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 230 Rest of Latin America Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 231 Rest of Latin America Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 232 MEA Decentralised Clinical Trials Market Forecast 2022-2032 (US$ million, AGR%)
Figure 233 MEA Decentralised Clinical Trials Market Forecast by Country 2022-2032 (US$ million)
Figure 234 MEA Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million)
Figure 235 MEA Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 236 MEA Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 237 MEA Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 238 MEA Decentralised Clinical Trials Market Forecast by Study Design, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 239 MEA Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million)
Figure 240 MEA Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 241 MEA Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 242 MEA Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 243 MEA Decentralised Clinical Trials Market Forecast by Indication, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 244 MEA Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million)
Figure 245 MEA Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "V" Shaped Recovery
Figure 246 MEA Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "U" Shaped Recovery
Figure 247 MEA Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "W" Shaped Recovery
Figure 248 MEA Decentralised Clinical Trials Market Forecast by End-User, 2022-2032 (US$ million): "L" Shaped Recovery
Figure 249 South Africa Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 250 South Africa Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 251 South Africa Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 252 South Africa Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 253 South Africa Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 254 GCC Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 255 GCC Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 256 GCC Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 257 GCC Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 258 GCC Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 259 Rest of MEA Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 260 Rest of MEA Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “V” Shaped Recovery
Figure 261 Rest of MEA Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “U” Shaped Recovery
Figure 262 Rest of MEA Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “W” Shaped Recovery
Figure 263 Rest of MEA Decentralised Clinical Trials Market Forecast, 2022-2032 (US$ million, AGR%): “L” Shaped Recovery
Figure 264 Decentralised Clinical Trials Market: Company Share Analysis, 2021
Figure 265 IQVIA Holdings Inc.: Net Revenue, 2016-2020 (US$ million, AGR%)
Figure 266 IQVIA Holdings Inc.: Regional Revenue Shares, 2020
Figure 267 IQVIA Holdings Inc.: Segmental Revenue Shares, 2020
Figure 268 ICON PLC: Net Revenue, 2016-2020 (US$ million, AGR%)
Figure 269 ICON PLC: Regional Revenue Shares, 2020
Figure 270 Laboratory Corporation of America Holdings (Covance, Inc.): Net Revenue, 2016-2020 (US$ million, AGR%)
Figure 271 Laboratory Corporation of America Holdings (Covance Inc.): Regional Revenue Shares, 2020
Figure 272 Laboratory Corporation of America Holdings (Covance Inc.): Segmental Revenue Shares, 2020
Figure 273 Dassault SystemesSE: Net Revenue, 2016-2020 (US$ million, AGR%)
Figure 274 Dassault SystemesSE: Regional Revenue Shares, 2020
Figure 275 Dassault SystemesSE: Segmental Revenue Shares, 2020
Figure 276 Oracle Corporation: Net Revenue, 2016-2020 (US$ million, AGR%)
Figure 277 Oracle Corporation: Regional Revenue Shares, 2020
Figure 278 Oracle Corporation: Segmental Revenue Shares, 2020
Figure 279 Syneos Health: Net Revenue, 2016-2020 (US$ million, AGR%)
Figure 280 Syneos Health: Regional Revenue Shares, 2020
Figure 281 Syneos Health: Segmental Revenue Shares, 2020
Figure 282 PPD, Inc.: Net Revenue, 2016-2020 (US$ million, AGR%)
Figure 283 PPD, Inc.: Regional Revenue Shares, 2020
Figure 284 PPD, Inc.: Segmental Revenue Shares, 2020
Figure 285 Ergomed PLC: Net Revenue, 2016-2020 (US$ million, AGR%)
Figure 286 Ergomed PLC: Regional Revenue Shares, 2020
Figure 287 Ergomed PLC: Segmental Revenue Shares, 2020
Figure 288 Global Decentralised Clinical Trials Market Share Forecast by Study Design, 2022, 2027, & 2032 (%)
Figure 289 Global Decentralised Clinical Trials Market Share Forecast by Region, 2022, 2027, 2032 (%)
CASTOR EDC
Clinical Ink, Inc. (G. I. Partners)
CLOUDZBY, INC.
Dassault SystemesSE (Medidata Solutions, Inc.)
ERGOMED PLC
Florence Healthcare, Inc.
ICON PLC
IQVIA Holdings, Inc.
Laboratory Corporation of America Holdings (Covance, Inc.)
Medable, Inc.
Medrio Inc.
Oracle Corporation
Parexel International Corporation
Pharmaceutical Product Development, Inc.
Syneos Health
List of Companies Mentioned in the Report
(FMD)
Aastrom Research International
AB Sciex
ABC Laboratories, Inc
ABF Pharmaceutical Services
Abingdon Life Sciences
Abridge
Absorption Systems
Accell Clinical Research
Accelovance
Accelsiors
Accium Biosciences
Accovion
Accumedix, Inc
Accutest Research Organization
Aclires
ACM
ACORN CRO
ACR Image Metrix
Acronet
aCROnordic A/S
Akos
Akrios BioDevelopment International
Albuquerque Clinical Trials (ACT)
AlcheraBio
Alembic
Aliora
Alliance Research Group
Allied Research International
Allphase
AlphaGenesis Incorporated (AGI)
and Consulting
Assign Group
Aviva Fridman Clinical Trials
Axio Research
AXIS Clinicals
AXON
Axon CRO
Axon Medchem
B&C Group
BASi
BBK
Beardsworth
Belnico
Beltas
Biofortis (Silliker)
BioStat International (BSI)
Biotoxtech Co., LTD
Biotrial
Biotrofix
Biovail Contract Research
Biovantix
Blue Sky Biotech
Boston MedTech Advisors
BPI Service Gmbh – Pooling Project Pharmacovigilance
Bradstreet Clinical Research
Bri Biopharmaceutical Research
Catalyst Clinical Services
CATO Research
CCDRD
CCS Associates
Cedarburg Hauser Pharmaceuticals
Celerion
CEMO
Centralabs Clinical Research, Inc (USA)
Cepha
ClinAssure
ClinAudits
ClinBay
Clindatrix
Clinesian Research Management
Clinimetrics
Cogtest Inc
Comac Medical
Commonwealth Biotechnologies
CPR
Cronova
Cropha CRO
Crown CRO
CTO Clinical Trial Operations
CTTI (Clinical Trials TEVA Israel)
Cu-Tech
Cyanta
D2L
Datapharm Australia
Dava Oncology
Davinci Biomedical Research Products, Inc.
DaVita Clinical Research
Dedicated Phase I, Inc.
DevDx Clinical
Developharma
DIL Limited
Dimension Research
Dimensione Ricerca (DR Group)
Discovery Research International (DRI)
Egeen International
Eliapharma Services Inc.
Emergo Group
Emerson Resources
Eminent Research Systems, Inc.
Emissary
Encorium
Endpoint
Ephoran
EpiStat Research
eResearchTechnology, Inc (ERT)
Ergomed
Estern Medical
FOVEA Group
Fractals Clinical Research
GSTT Emergency Scientific and Medical Services
HungaroTrial
Huntingdon Life Sciences (UK)
Hurley Consulting
Hylae Clinical Research
Hyperion Biotechnology
IATEC
ICON Clinical
ICRC-Weyer (Independent Clinical Research Consulting)
ICTS
IFE Baltic UAB
iGATE Clinical Research International
Imperial Clinical Research Services
in Good Clinical Practice)
Inamed
INC Research
Intrinsic Imaging LLC
InVentiv Clinical
iResearch Limited
Iris Pharma
IRW Consulting
IST
Jai Research Foundation (JRF)
JANIX
Jasper
Java Clinical
JSS Medical Research
JSW Life Sciences
Limited
MediCroStar
Medidata Solutions, Inc
Mediolanum Cardio Resesarch (MCR)
Mediprobe Research Inc
Mediscis
Mediserv
Monitoring Force
MPI Research
MSOURCE
MTZ Clinical Research
Nagy Research
Ora Clinical
Oracle
Orange Lifesciences
Orbis Data Solutions
Orion Clinical Services
Oroxcell
Pharm-Olam International (POI)
Pharmaffiliates
Pharmahungary
PharmaIntel
Pharmalog
Pharmalys
Pharmanet
PharmaPart
Pharmatech Oncology
Pharmatek Laboratories, Inc
PharmaTrials, Inc
PharmStats
PharSafer Associates Ltd
Pharsight Corporation
Phaze SA
PHDS Healthcare Research
Phidea /Marvin
PHT Corporation (USA)
Physiogenix
Piedmont
Pierrel
ProInnovera
Prologue Research International
Promedica International
PROMetrika
ProTrials
Radiant Research
RCC Laboratories
RCRI
Recerca Clinica
Registrat-MAPI
Regulatory Clinical Research Institute, Inc (RCRI)
Rejuvendus Clinical Research
Reliance Clinical Research Services (Reliance Life Sciences)
RenaSci
Rephartox
Sanmar Speciality Chemicals Ltd
Service)
SGS Lifesciences
Shandon Clinical Trials
ShangPharma
Shin Nippon Biomedical Laboratories
Siesta Group
Simbec Research
Sinclair Research
Siri Technologies
Siro Clinpharm
SLG (China-CRO)
Smerud Medical Research
SMO-USA
Symphony Pharma Life Sciences
SYNARC/CCBR
Syncare Clinical Services
Synchron
Synexus
SynRG (Synergy Research Group (SRG))
Synteract
Syreon
Sysdata Consulting
TAKE Solutions
TargetHealth
Tata Indicom Ebu (India)
TCG Lifesciences Limited (formerly known as Chembiotek)
Tech Observer
Tetrial, LLC
TGA Sciences, Inc
The Clinical Trial Company (TCTC)
The CRO Group, Inc.
The Patient Recruiting Agency (TPRA)
Universal Research Group
Vital Systems Inc
Vivo Bio Tech Limited
VPS-CRO (VenturePharm Services)
Washington Biotechnology, Inc
Watermark Research Partners
WCCT (West Coast Clinical Trials)
Westat
Winicker Norimed
Worldwide Clinical Trials (WWCT, WCT, CEDRA)
WuXi AppTec
Xceleron
Xendo
ZeinCRO
ZM Company
List of Organizations Mentioned in the Report
ACRF Centre for Advanced Decentralized Clinical Trials
Agencia Nacional de Vigiloncia Sanitaria (ANVISA)
American Cancer Society (ACS)
Center for Biologics Evaluation and Research (CBER)
Center for Devices and Radiological Health (CDRH)
Center of Excellence in Immunology (CEI)
Centers for Disease Control and Prevention (CDC)
Centers for Medicare & Medicaid Services programs
European Medicines Agency (EMA)
European Union
Experimental Transplantation & Immunotherapy Branch at CCR
Food and Drug Administration (FDA)
French Government
Government of Japan
Health Ministry (Italy)
Indian Council of Medical Research (ICMR)
Institute for Healthcare Improvement (IHI)
International Agency for Research on Cancer (IARC)
Italian Industry Group Farmindustria
Japanese Ministry of Health, Labour and Welfare
Laboratory of Tumor Immunology and Biology at CCR
Medicines and Healthcare Products Regulatory Agency (MHRA)
Ministry of Health, Labour & Welfare (MHLW)
National Cancer Institute (NCI)
National Cancer Institute's Center for Cancer Research (CCR)
National Development and Reform Commission (NDRC)
National Institutes of Health (NIH)
NCBI
NCI's Division of Cancer Biology's Cancer Immunology, Hematology, and Etiology Branch (CIHEB)
The Therapeutic Goods Administration
United Nations (UN)
University of Connecticut
World Health Organization (WHO)
World Population Prospects
Download sample pages
Complete the form below to download your free sample pages for Decentralized Clinical Trials Market Report 2022-2032
Related reports
-

Clinical Trial Packaging and Labelling Market Report 2023-2033
The global Clinical Trial Packaging and Labelling market is projected to grow at a CAGR of 6.08% by 2033....Full DetailsPublished: 03 January 2023 -

Clinical Trial Supply and Logistics Market for Pharma 2021-2031
Digital innovation in clinical trials and renewed focus on home production will ensure a high growth market for supply chain...Full DetailsPublished: 08 July 2021 -

Clinical Trial Supplies Market Report 2021-2031
Deviating from protocols raises the danger of missing or delaying data collection from current investigations. This highlights the growing importance...Full DetailsPublished: 11 October 2021 -

Pharma Clinical Trial Services Market and Industry Forecast 2020-2030
The pharma clinical trial services market is expected to grow at a CAGR of 10% in the first half of...
Full DetailsPublished: 20 March 2020 -

Vaccine Sales Market Report 2022-2032
Rising epidemic potential, a growing emphasis on therapeutic vaccines, and new markets are projected to provide considerable growth prospects for...Full DetailsPublished: 21 April 2022 -

Clinical Trial Supplies Market Report 2023-2033
The global Clinical Trial Supplies market is projected to grow at a CAGR of 9.32% by 2033...Full DetailsPublished: 31 January 2023 -

eClinical Solutions Market Report 2023-2033
The global eClinical Solutions market is projected to grow at a CAGR of 13.09% by 2033...Full DetailsPublished: 17 March 2023 -

Clinical Trials Materials Storage and Distribution Market Report 2020-2030
The global Clinical Trials Materials Storage and Distribution market for pharma is estimated to have reach US$xyz million in 2020...
Full DetailsPublished: 16 June 2020 -

Deep Frozen Packaging Logistics Market Report 2022-2032
The desire for various types of medications to assist combat a growing number of chronic diseases & lifestyle-related ailments is...Full DetailsPublished: 14 January 2022 -

Pharmaceutical Contract Manufacturing Market Report 2022-2032
The major driving factor contributing towards the growth of pharmaceutical contract manufacturing market are increase in investments in pharmaceutical R&D,...Full DetailsPublished: 13 April 2022
Download sample pages
Complete the form below to download your free sample pages for Decentralized Clinical Trials Market Report 2022-2032
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Don’t Miss Out!
Subscribe to receive the latest Industry news, trending insight and analysis straight to your inbox.Choose your preferences:
Latest Pharma news
Retinal Gene Therapy Market
The global Retinal Gene Therapy market is projected to grow at a CAGR of 9.6% by 2034
26 July 2024
HIV Drugs and Injectables Market
The global HIV Drugs & Injectables market is projected to grow at a CAGR of 4.6 % by 2034
24 July 2024
Digital Twin Technology in Pharmaceutical Manufacturing Market
The global Digital Twin Technology in Pharmaceutical Manufacturing market is projected to grow at a CAGR of 31.3% by 2034
23 July 2024
Specialty Pharma Market
The global Specialty Pharma market is projected to grow at a CAGR of 7.5% by 2034
22 July 2024


















