Industries > Pharma > Clinical Trial Supplies Market Report 2023-2033
Clinical Trial Supplies Market Report 2023-2033
Forecasts by Location (Offshore Clinical Site, Domestic Clinical Site), by Type (Small Molecule Products, Biologic Products, Medical Devices), by Services (Manufacturing (IMPs and INDs, Placebos, Assays and Test Kits, Others), Logistics & Distribution (Cold Chain Distribution, Non-cold Chain), Storage & Retention, Packaging and Labelling, Comparator Sourcing, Other), by Therapeutic Areas (Oncology, CNS and Mental Disorders, Cardiovascular Disease (CVD), Infectious Disease, Immunology Disease, Blood Disorders, Metabolic Disorders, Digestive Disorders, Other), by Phase (Phase-I, Phase-II, Phase-III, Phase-IV), by End-user (Contract Research Organisations, Pharmaceutical and Biotechnology Companies, Medical Device Companies, Other) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Impact and Recovery Pattern Analysis
The Clinical Trial Supplies Market Report 2023-2033: This report will prove invaluable to leading firms striving for new revenue pockets if they wish to better understand the industry and its underlying dynamics. It will be useful for companies that would like to expand into different industries or to expand their existing operations in a new region.
Increasing Demand for Clinical Trial Outsourcing is driving the Demand for Clinical Trial Supplies Market
Downsizing and cost-cutting are important factors for large and mid-size pharmaceutical companies to outsource clinical trials, as CRO expertise in a specific therapeutic in a market where the company lacks experience with regulatory processes, operational expertise, and the ability to use manpower for other activities at the time of increasing workload. Furthermore, due to the increasing process complexity and cost of clinical studies, pharmaceutical manufacturers have been forced to outsource clinical trial supplies and supply them to research sites for reducing cost and time. Clinical supply firms are concentrating on data sharing to assist them build a solid supply chain for clinical consumables during clinical trials. This factor will have a favourable impact on the revenue growth of the clinical trial supplies market throughout the forecast period.
Unavailability of Storage Facilities in across Multiple Nations May Hamper the Expansion Clinical Trial Supplies Market
Certain clinical materials must be stored under specific climatic conditions, and such facilities are critical before conducting clinical studies. However, in countries such as Mexico, Brazil, and others, lack of clinical supply storage facilities and infrastructure may impede market acceptance of clinical trial supplies services. It is critical, for example, to determine early on if important components of the cold chain must be held in controlled temperature facilities if things are stored. Transportation of clinical supplies from cold storage to clinical sites is difficult, which is projected to limit revenue growth in this market to some extent.
What Questions Should You Ask before Buying a Market Research Report?
• How is the clinical trial supplies market evolving?
• What is driving and restraining the clinical trial supplies market?
• How will each clinical trial supplies submarket segment grow over the forecast period and how much revenue will these submarkets account for in 2033?
• How will the market shares for each clinical trial supplies submarket develop from 2023 to 2033?
• What will be the main driver for the overall market from 2023 to 2033?
• Will leading clinical trial supplies markets broadly follow the macroeconomic dynamics, or will individual national markets outperform others?
• How will the market shares of the national markets change by 2033 and which geographical region will lead the market in 2033?
• Who are the leading players and what are their prospects over the forecast period?
• What are the clinical trial supplies projects for these leading companies?
• How will the industry evolve during the period between 2023 and 2033? What are the implications of clinical trial supplies projects taking place now and over the next 10 years?
• Is there a greater need for product commercialisation to further scale the clinical trial supplies market?
• Where is the clinical trial supplies market heading and how can you ensure you are at the forefront of the market?
• What are the best investment options for new product and service lines?
• What are the key prospects for moving companies into a new growth path and C-suite?
You need to discover how this will impact the clinical trial supplies market today, and over the next 10 years:
• Our 423-page report provides 187 tables and 272 charts/graphs exclusively to you.
• The report highlights key lucrative areas in the industry so you can target them – NOW.
• It contains in-depth analysis of global, regional and national sales and growth.
• It highlights for you the key successful trends, changes and revenue projections made by your competitors.
This report tells you TODAY how the clinical trial supplies market will develop in the next 10 years, and in line with the variations in COVID-19 economic recession and bounce. This market is more critical now than at any point over the last 10 years.
Forecasts to 2033 and other analyses reveal commercial prospects
• In addition to revenue forecasting to 2033, our new study provides you with recent results, growth rates, and market shares.
• You will find original analyses, with business outlooks and developments.
• Discover qualitative analyses (including market dynamics, drivers, opportunities, restraints and challenges), cost structure, impact of rising clinical trial supplies prices and recent developments.
This report includes data analysis and invaluable insight into how COVID-19 will affect the industry and your company. Four COVID-19 recovery patterns and their impact, namely, “V”, “L”, “W” and “U” are discussed in this report.
Segments Covered in the Report
Location
• Offshore Clinical Site
• Domestic Clinical Site
Type
• Small Molecule Products
• Biologic Products
• Medical Devices
Services
• Manufacturing
– IMPs and INDs
– Placebos
– Assays and Testkits
– Others
• Logistics & Distribution
– Cold Chain Distribution
– Non-cold Chain
• Storage & Retention
• Packaging and Labeling
• Comparator Sourcing
• Other CTS Services
Therapeutic Areas
• Oncology
• CNS and Mental Disorders
• Cardiovascular Disease (CVD)
• Infectious Disease
• Immunology Disease
• Blood Disorders
• Metabolic Disorders
• Digestive Disorders
• Other Therapeutic Areas
Phase
• Phase-I
• Phase-II
• Phase-III
• Phase-IV
End-User
• Contract Research Organizations
• Pharmaceutical and Biotechnology Companies
• Medical Device Companies
• Other End-Users
In addition to the revenue predictions for the overall world market and segments, you will also find revenue forecasts for five regional and 23 leading national markets:
North America
• U.S.
• Canada
Europe
• Germany
• UK
• France
• Italy
• Spain
• Russia
• Rest of Europe
Asia Pacific
• China
• India
• Japan
• South Korea
• Australia
• Rest of Asia Pacific
Latin America
• Brazil
• Mexico
• Rest of Latin America
MEA
• South Africa
• Saudi Arabia
• Turkey
• UAE
• Rest of MEA
Need industry data? Please contact us today.
The report also includes profiles and for some of the leading companies in the Clinical Trial Supplies Market, 2023 to 2033, with a focus on this segment of these companies’ operations.
Leading companies and the potential for market growth
• ADAllen Pharma
• AmerisourceBergen Corporation
• Ancillare
• Biocair
• Catalent, Inc.
• Clinigen Group plc
• Coghlan Group
• COREX Logistics
• DHL
• Durbin
• Endpoint Clinical
• Eurofins Scientific SE
• Inizio (UDG Healthcare plc)
• IQVIA Inc.
• KLIFO
• Liveo Research
• Lonza
• Marken (A UPS Company)
• Movianto
• Myonex
• N-SIDE
• Parexel
• PCI Pharma Services
• Piramal Pharma Solutions
• Recipharm AB
• Rubicon Research Pvt S.A.
• Seveillar Clinical Supplies Services
• Sharp Corporation
• SIRO Clinpharm
• Thermo Fisher Scientific Inc.
Overall world revenue for Clinical Trial Supplies Market, 2023 to 2033 in terms of value the market will surpass US$ 2,819.2 million in 2023, our work calculates. We predict strong revenue growth through to 2033. Our work identifies which organizations hold the greatest potential. Discover their capabilities, progress, and commercial prospects, helping you stay ahead.
How will the Clinical Trial Supplies Market, 2023 to 2033 report help you?
In summary, our 420+ page report provides you with the following knowledge:
• Revenue forecasts to 2033 for Clinical Trial Supplies Market, 2023 to 2033, with forecasts for location, type, services, therapeutic areas, phase, and end-user, each forecast at a global and regional level – discover the industry’s prospects, finding the most lucrative places for investments and revenues.
• Revenue forecasts to 2033 for five regional and 19 key national markets – See forecasts for the Clinical Trial Supplies Market, 2023 to 2033 market in North America, Europe, Asia-Pacific, Latin America, and MEA. Also forecasted is the market in the US, Canada, Mexico, Brazil, Germany, France, UK, Italy, China, India, Japan, and Australia among other prominent economies.
• Prospects for established firms and those seeking to enter the market – including company profiles for 30 of the major companies involved in the Clinical Trial Supplies Market, 2023 to 2033.
Find quantitative and qualitative analyses with independent predictions. Receive information that only our report contains, staying informed with invaluable business intelligence.
Information found nowhere else
With our new report, you are less likely to fall behind in knowledge or miss out on opportunities. See how our work could benefit your research, analyses, and decisions. Visiongain’s study is for everybody needing commercial analyses for the Clinical Trial Supplies Market, 2023 to 2033, market-leading companies. You will find data, trends and predictions.
To access the data contained in this document please email contactus@visiongain.com
Buy our report today Clinical Trial Supplies Market Report 2023-2033: Forecasts by Location (Offshore Clinical Site, Domestic Clinical Site), by Type (Small Molecule Products, Biologic Products, Medical Devices), by Services (Manufacturing (IMPs and INDs, Placebos, Assays and Test Kits, Others), Logistics & Distribution (Cold Chain Distribution, Non-cold Chain), Storage & Retention, Packaging and Labelling, Comparator Sourcing, Other), by Therapeutic Areas (Oncology, CNS and Mental Disorders, Cardiovascular Disease (CVD), Infectious Disease, Immunology Disease, Blood Disorders, Metabolic Disorders, Digestive Disorders, Other), by Phase (Phase-I, Phase-II, Phase-III, Phase-IV), by End-user (Contract Research Organisations, Pharmaceutical and Biotechnology Companies, Medical Device Companies, Other) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Impact and Recovery Pattern Analysis. Avoid missing out by staying informed – order our report now.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for a specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: contactus@visiongain.com
1.1 Objectives of the Study
1.2 Introduction to Clinical Trial Supplies Market
1.3 What This Report Delivers
1.4 Why You Should Read This Report
1.5 Key Questions Answered by This Analytical Report
1.6 Who is This Report for?
1.7 Methodology
1.7.1 Market Definitions
1.7.2 Market Evaluation & Forecasting Methodology
1.7.3 Data Validation
1.7.3.1 Primary Research
1.7.3.2 Secondary Research
1.8 Frequently Asked Questions (FAQs)
1.9 Associated Visiongain Reports
1.10 About Visiongain
2 Executive Summary
3 Market Overview
3.1 Key Findings
3.2 Market Dynamics
3.2.1 Market Driving Factors
3.2.1.1 Increasing Demand for Clinical Trial Outsourcing
3.2.1.2 Rising Demand for Biologics Propelling Growth of Cold Chain Supplies
3.2.1.3 Increasing Investments in the Development of Novel Pharmaceuticals
3.2.1.4 Technology Advancements are Propelling Market Expansion
3.2.2 Market Restraining
3.2.2.1 Availability of Storage Facilities in Each Country
3.2.2.2 Lack of Comparator Sourcing
3.2.2.3 High Costs Associated with Drug Developments
3.2.3 Market Opportunities
3.2.3.1 Increasing Opportunities for Emerging Markets
3.2.3.2 Cut in High Costs Expected to Propel Market Growth
3.2.3.3 Integration of Technical Solutions
3.3 COVID-19 Impact Analysis
3.4 PEST Analysis
3.4.1 Political Factors
3.4.2 Economic Factors
3.4.3 Social Factors
3.4.4 Technological Factors
4 Clinical Trial Supplies Market Analysis by Location
4.1 Key Findings
4.2 Location Segment: Market Attractiveness Index
4.3 Clinical Trial Supplies Market Size Estimation and Forecast by Location
4.4 Offshore Clinical Site
4.4.1 Market Size by Region, 2023-2033 (US$ Mn)
4.4.2 Market Share by Region, 2023 & 2033 (%)
4.5 Domestic Clinical Site
4.5.1 Market Size by Region, 2023-2033 (US$ Mn)
4.5.2 Market Share by Region, 2023 & 2033 (%)
5 Clinical Trial Supplies Market Analysis by Type
5.1 Key Findings
5.2 Type Segment: Market Attractiveness Index
5.3 Clinical Trial Supplies Market Size Estimation and Forecast by Type
5.4 Small Molecule Products
5.4.1 Market Size by Region, 2023-2033 (US$ Mn)
5.4.2 Market Share by Region, 2023 & 2033 (%)
5.5 Biologic Products
5.5.1 Market Size by Region, 2023-2033 (US$ Mn)
5.5.2 Market Share by Region, 2023 & 2033 (%)
5.6 Medical Devices
5.6.1 Market Size by Region, 2023-2033 (US$ Mn)
5.6.2 Market Share by Region, 2023 & 2033 (%)
6 Clinical Trial Supplies Market Analysis by Services
6.1 Key Findings
6.2 Services Segment: Market Attractiveness Index
6.3 Clinical Trial Supplies Market Size Estimation and Forecast by Services
6.4 Manufacturing
6.4.1 Market Size by Region, 2023-2033 (US$ Mn)
6.4.2 Market Share by Region, 2023 & 2033 (%)
6.4.3 IMPs and INDs
6.4.4 Market Size by Region, 2023-2033 (US$ Mn)
6.4.5 Market Share by Region, 2023 & 2033 (%)
6.4.6 Placebos
6.4.7 Market Size by Region, 2023-2033 (US$ Mn)
6.4.8 Market Share by Region, 2023 & 2033 (%)
6.4.9 Assays and Test kits
6.4.10 Market Size by Region, 2023-2033 (US$ Mn)
6.4.11 Market Share by Region, 2023 & 2033 (%)
6.4.12 Others
6.4.13 Market Size by Region, 2023-2033 (US$ Mn)
6.4.14 Market Share by Region, 2023 & 2033 (%)
6.5 Logistics & Distribution
6.5.1 Market Size by Region, 2023-2033 (US$ Mn)
6.5.2 Market Share by Region, 2023 & 2033 (%)
6.5.3 Cold Chain Distribution
6.5.4 Market Size by Region, 2023-2033 (US$ Mn)
6.5.5 Market Share by Region, 2023 & 2033 (%)
6.5.6 Non-cold Chain
6.5.7 Market Size by Region, 2023-2033 (US$ Mn)
6.5.8 Market Share by Region, 2023 & 2033 (%)
6.6 Storage & Retention
6.6.1 Market Size by Region, 2023-2033 (US$ Mn)
6.6.2 Market Share by Region, 2023 & 2033 (%)
6.7 Packaging and Labelling
6.7.1 Market Size by Region, 2023-2033 (US$ Mn)
6.7.2 Market Share by Region, 2023 & 2033 (%)
6.8 Comparator Sourcing
6.8.1 Market Size by Region, 2023-2033 (US$ Mn)
6.8.2 Market Share by Region, 2023 & 2033 (%)
6.9 Other CTS Services
6.9.1 Market Size by Region, 2023-2033 (US$ Mn)
6.9.2 Market Share by Region, 2023 & 2033 (%)
7 Clinical Trial Supplies Market Analysis by Therapeutic Areas
7.1 Key Findings
7.2 Therapeutic Areas Segment: Market Attractiveness Index
7.3 Clinical Trial Supplies Market Size Estimation and Forecast by Therapeutic Areas
7.4 Oncology
7.4.1 Market Size by Region, 2023-2033 (US$ Mn)
7.4.2 Market Share by Region, 2023 & 2033 (%)
7.5 CNS and Mental Disorders
7.5.1 Market Size by Region, 2023-2033 (US$ Mn)
7.5.2 Market Share by Region, 2023 & 2033 (%)
7.6 Cardiovascular Disease (CVD)
7.6.1 Market Size by Region, 2023-2033 (US$ Mn)
7.6.2 Market Share by Region, 2023 & 2033 (%)
7.7 Infectious Disease
7.7.1 Market Size by Region, 2023-2033 (US$ Mn)
7.7.2 Market Share by Region, 2023 & 2033 (%)
7.8 Immunological Diseases
7.8.1 Market Size by Region, 2023-2033 (US$ Mn)
7.8.2 Market Share by Region, 2023 & 2033 (%)
7.9 Blood Disorders
7.9.1 Market Size by Region, 2023-2033 (US$ Mn)
7.9.2 Market Share by Region, 2023 & 2033 (%)
7.10 Metabolic Disorders
7.10.1 Market Size by Region, 2023-2033 (US$ Mn)
7.10.2 Market Share by Region, 2023 & 2033 (%)
7.11 Digestive Disorders
7.11.1 Market Size by Region, 2023-2033 (US$ Mn)
7.11.2 Market Share by Region, 2023 & 2033 (%)
7.12 Other Therapeutic Areas
7.12.1 Market Size by Region, 2023-2033 (US$ Mn)
7.12.2 Market Share by Region, 2023 & 2033 (%)
8 Clinical Trial Supplies Market Analysis by Phase
8.1 Key Findings
8.2 Phase Segment: Market Attractiveness Index
8.3 Clinical Trial Supplies Market Size Estimation and Forecast by Phase
8.4 Phase-I
8.4.1 Market Size by Region, 2023-2033 (US$ Mn)
8.4.2 Market Share by Region, 2023 & 2033 (%)
8.5 Phase-II
8.5.1 Market Size by Region, 2023-2033 (US$ Mn)
8.5.2 Market Share by Region, 2023 & 2033 (%)
8.6 Phase-III
8.6.1 Market Size by Region, 2023-2033 (US$ Mn)
8.6.2 Market Share by Region, 2023 & 2033 (%)
8.7 Phase-IV
8.7.1 Market Size by Region, 2023-2033 (US$ Mn)
8.7.2 Market Share by Region, 2023 & 2033 (%)
9 Clinical Trial Supplies Market Analysis by End-User
9.1 Key Findings
9.2 End-User Segment: Market Attractiveness Index
9.3 Clinical Trial Supplies Market Size Estimation and Forecast by End-User
9.4 Contract Research Organizations
9.4.1 Market Size by Region, 2023-2033 (US$ Mn)
9.4.2 Market Share by Region, 2023 & 2033 (%)
9.5 Pharmaceutical and Biotechnology Companies
9.5.1 Market Size by Region, 2023-2033 (US$ Mn)
9.5.2 Market Share by Region, 2023 & 2033 (%)
9.6 Medical Device Companies
9.6.1 Market Size by Region, 2023-2033 (US$ Mn)
9.6.2 Market Share by Region, 2023 & 2033 (%)
9.7 Other End-Users
9.7.1 Market Size by Region, 2023-2033 (US$ Mn)
9.7.2 Market Share by Region, 2023 & 2033 (%)
10 Clinical Trial Supplies Market Analysis by Region
10.1 Key Findings
10.2 Regional Market Size Estimation and Forecast
11 North America Clinical Trial Supplies Market Analysis
11.1 Key Findings
11.2 North America Clinical Trial Supplies Market Attractiveness Index
11.3 North America Clinical Trial Supplies Market by Country, 2023, 2028 & 2033 (US$ bn)
11.4 North America Clinical Trial Supplies Market Size Estimation and Forecast by Country
11.5 North America Clinical Trial Supplies Market Size Estimation and Forecast by Location
11.6 North America Clinical Trial Supplies Market Size Estimation and Forecast by Type
11.7 North America Clinical Trial Supplies Market Size Estimation and Forecast by Services
11.7.1 North America Clinical Trial Supplies Market Size Estimation and Forecast by Manufacturing
11.7.2 North America Clinical Trial Supplies Market Size Estimation and Forecast by Logistics & Distribution
11.8 North America Clinical Trial Supplies Market Size Estimation and Forecast by Therapeutic Areas
11.9 North America Clinical Trial Supplies Market Size Estimation and Forecast by Phase
11.10 North America Clinical Trial Supplies Market Size Estimation and Forecast by End-User
11.11 U.S. Clinical Trial Supplies Market Analysis
11.12 Canada Clinical Trial Supplies Market Analysis
12 Europe Clinical Trial Supplies Market Analysis
12.1 Key Findings
12.2 Europe Clinical Trial Supplies Market Attractiveness Index
12.3 Europe Clinical Trial Supplies Market by Country, 2023, 2028 & 2033 (US$ bn)
12.4 Europe Clinical Trial Supplies Market Size Estimation and Forecast by Country
12.5 Europe Clinical Trial Supplies Market Size Estimation and Forecast by Location
12.6 Europe Clinical Trial Supplies Market Size Estimation and Forecast by Type
12.7 Europe Clinical Trial Supplies Market Size Estimation and Forecast by Services
12.7.1 Europe Clinical Trial Supplies Market Size Estimation and Forecast by Manufacturing
12.7.2 Europe Clinical Trial Supplies Market Size Estimation and Forecast by Logistics & Distribution
12.8 Europe Clinical Trial Supplies Market Size Estimation and Forecast by Therapeutic Areas
12.9 Europe Clinical Trial Supplies Market Size Estimation and Forecast by Phase
12.10 Europe Clinical Trial Supplies Market Size Estimation and Forecast by End-User
12.11 Germany Clinical Trial Supplies Market Analysis
12.12 UK Clinical Trial Supplies Market Analysis
12.13 France Clinical Trial Supplies Market Analysis
12.14 Italy Clinical Trial Supplies Market Analysis
12.15 Spain Clinical Trial Supplies Market Analysis
12.16 Russia Clinical Trial Supplies Market Analysis
12.17 Rest of Europe Clinical Trial Supplies Market Analysis
13 Asia Pacific Clinical Trial Supplies Market Analysis
13.1 Key Findings
13.2 Asia Pacific Clinical Trial Supplies Market Attractiveness Index
13.3 Asia Pacific Clinical Trial Supplies Market by Country, 2023, 2028 & 2033 (US$ bn)
13.4 Asia Pacific Clinical Trial Supplies Market Size Estimation and Forecast by Country
13.5 Asia Pacific Clinical Trial Supplies Market Size Estimation and Forecast by Location
13.6 Asia Pacific Clinical Trial Supplies Market Size Estimation and Forecast by Type
13.7 Asia Pacific Clinical Trial Supplies Market Size Estimation and Forecast by Services
13.7.1 Asia Pacific Clinical Trial Supplies Market Size Estimation and Forecast by Manufacturing
13.7.2 Asia Pacific Clinical Trial Supplies Market Size Estimation and Forecast by Logistics & Distribution
13.8 Asia Pacific Clinical Trial Supplies Market Size Estimation and Forecast by Therapeutic Areas
13.9 Asia Pacific Clinical Trial Supplies Market Size Estimation and Forecast by Phase
13.10 Asia Pacific Clinical Trial Supplies Market Size Estimation and Forecast by End-User
13.11 China Clinical Trial Supplies Market Analysis
13.12 India Clinical Trial Supplies Market Analysis
13.13 Japan Clinical Trial Supplies Market Analysis
13.14 South Korea Clinical Trial Supplies Market Analysis
13.15 Australia Clinical Trial Supplies Market Analysis
13.16 Rest of Asia-Pacific Clinical Trial Supplies Market Analysis
14 Latin America Clinical Trial Supplies Market Analysis
14.1 Key Findings
14.2 Latin America Clinical Trial Supplies Market Attractiveness Index
14.3 Latin America Clinical Trial Supplies Market by Country, 2023, 2028 & 2033 (US$ bn)
14.4 Latin America Clinical Trial Supplies Market Size Estimation and Forecast by Country
14.5 Latin America Clinical Trial Supplies Market Size Estimation and Forecast by Location
14.6 Latin America Clinical Trial Supplies Market Size Estimation and Forecast by Type
14.7 Latin America Clinical Trial Supplies Market Size Estimation and Forecast by Services
14.7.1 Latin America Clinical Trial Supplies Market Size Estimation and Forecast by Manufacturing
14.7.2 Latin America Clinical Trial Supplies Market Size Estimation and Forecast by Logistics & Distribution
14.8 Latin America Clinical Trial Supplies Market Size Estimation and Forecast by Therapeutic Areas
14.9 Latin America Clinical Trial Supplies Market Size Estimation and Forecast by Phase
14.10 Latin America Clinical Trial Supplies Market Size Estimation and Forecast by End-User
14.11 Brazil Clinical Trial Supplies Market Analysis
14.12 Mexico Clinical Trial Supplies Market Analysis
14.13 Rest of Latin America Clinical Trial Supplies Market Analysis
15 MEA Clinical Trial Supplies Market Analysis
15.1 Key Findings
15.2 MEA Clinical Trial Supplies Market Attractiveness Index
15.3 MEA Clinical Trial Supplies Market by Country, 2023, 2028 & 2033 (US$ bn)
15.4 MEA Clinical Trial Supplies Market Size Estimation and Forecast by Country
15.5 MEA Clinical Trial Supplies Market Size Estimation and Forecast by Location
15.6 MEA Clinical Trial Supplies Market Size Estimation and Forecast by Type
15.7 MEA Clinical Trial Supplies Market Size Estimation and Forecast by Services
15.7.1 MEA Clinical Trial Supplies Market Size Estimation and Forecast by Manufacturing
15.7.2 MEA Clinical Trial Supplies Market Size Estimation and Forecast by Logistics & Distribution
15.8 MEA Clinical Trial Supplies Market Size Estimation and Forecast by Therapeutic Areas
15.9 MEA Clinical Trial Supplies Market Size Estimation and Forecast by Phase
15.10 MEA Clinical Trial Supplies Market Size Estimation and Forecast by End-User
15.11 South Africa Clinical Trial Supplies Market Analysis
15.12 Saudi Arabia Clinical Trial Supplies Market Analysis
15.13 Turkey Clinical Trial Supplies Market Analysis
15.14 UAE Clinical Trial Supplies Market Analysis
15.15 Rest of MEA Clinical Trial Supplies Market Analysis
16 Company Profiles
16.1 Competitive Landscape, 2021
16.2 Strategic Outlook
16.3 Thermo Fisher Scientific Inc.
16.3.1 Company Snapshot
16.3.2 Company Overview
16.3.3 Financial Analysis
16.3.3.1 Net Revenue, 2017-2021
16.3.3.2 R&D, 2017-2021
16.3.3.3 Gross Profit, 2017-2021
16.3.3.4 Regional Market Shares, 2021
16.3.4 Product Benchmarking
16.3.5 Strategic Outlook
16.4 Catalent, Inc.
16.4.1 Company Snapshot
16.4.2 Company Overview
16.4.3 Financial Analysis
16.4.3.1 Net Revenue, 2017-2021
16.4.3.2 EBITDA, 2017-2021
16.4.3.3 Net Income, 2017-2021
16.4.3.4 Regional Market Shares, 2021
16.4.4 Product Benchmarking
16.4.5 Strategic Outlook
16.5 Piramal Pharma Solutions
16.5.1 Company Snapshot
16.5.2 Company Overview
16.5.3 Product Benchmarking
16.5.4 Strategic Outlook
16.6 Eurofins Scientific SE
16.6.1 Company Snapshot
16.6.2 Company Overview
16.6.3 Financial Analysis
16.6.3.1 Net Revenue, 2017-2021
16.6.3.2 Operating Income, 2017-2021
16.6.3.3 EBITDA, 2017-2021
16.6.3.4 Net Income, 2017-2021
16.6.3.5 Regional Market Shares, 2021
16.6.4 Product Benchmarking
16.7 Lonza
16.7.1 Company Snapshot
16.7.2 Company Overview
16.7.3 Financial Analysis
16.7.3.1 Net Revenue, 2017-2021
16.7.3.2 EBIT, 2017-2021
16.7.3.3 Gross Profit, 2017-2021
16.7.4 Product Benchmarking
16.7.5 Strategic Outlook
16.8 Clinigen Group plc
16.8.1 Company Snapshot
16.8.2 Company Overview
16.8.3 Financial Analysis
16.8.3.1 Net Revenue, 2017-2021
16.8.3.2 EBITDA, 2017-2021
16.8.3.3 Net Income, 2017-2021
16.8.4 Product Benchmarking
16.8.5 Strategic Outlook
16.9 AmerisourceBergen Corporation
16.9.1 Company Snapshot
16.9.2 Company Overview
16.9.3 Financial Analysis
16.9.3.1 Net Revenue, 2017-2021
16.9.3.2 Operating Income, 2017-2021
16.9.3.3 EBITDA, 2017-2021
16.9.3.4 Net Income, 2017-2021
16.9.4 Product Benchmarking
16.9.5 Strategic Outlook
16.10 DHL
16.10.1 Company Snapshot
16.10.2 Company Overview
16.10.3 Financial Analysis
16.10.3.1 Net Revenue, 2017-2021
16.10.3.2 EBITDA, 2017-2021
16.10.3.3 Gross Profit, 2017-2021
16.10.3.4 Net Income, 2017-2021
16.10.3.5 Operating Income, 2017-2021
16.10.3.6 Regional Market Share, 2017-2021
16.10.4 Product Benchmarking
16.10.5 Strategic Outlook
16.11 Sharp Corporation
16.11.1 Company Snapshot
16.11.2 Company Overview
16.11.3 Financial Analysis
16.11.3.1 Net Revenue, 2017-2021
16.11.3.2 EBITDA, 2017-2021
16.11.3.3 Net Income, 2017-2021
16.11.3.4 Operating Income, 2017-2021
16.11.3.5 Gross Profit, 2017-2021
16.11.4 Product Benchmarking
16.11.5 Strategic Outlook
16.12 IQVIA Inc.
16.12.1 Company Snapshot
16.12.2 Company Overview
16.12.3 Financial Analysis
16.12.3.1 Net Revenue, 2017-2021
16.12.3.2 EBITDA, 2017-2021
16.12.3.3 Gross Profit, 2017-2021
16.12.3.4 Net Income, 2017-2021
16.12.3.5 Operating Income, 2017-2021
16.12.3.6 Regional Market Share, 2017-2021
16.12.4 Product Benchmarking
16.12.5 Strategic Outlook
16.13 Parexel
16.13.1 Company Snapshot
16.13.2 Company Overview
16.13.3 Product Benchmarking
16.13.4 Strategic Outlook
16.14 Biocair
16.14.1 Company Snapshot
16.14.2 Company Overview
16.14.3 Product Benchmarking
16.14.4 Strategic Outlook
16.15 Liveo Research
16.15.1 Company Snapshot
16.15.2 Company Overview
16.15.3 Product Benchmarking
16.15.4 Strategic Outlook
16.16 Marken (A UPS Company)
16.16.1 Company Snapshot
16.16.2 Company Overview
16.16.3 Product Benchmarking
16.16.4 Strategic Outlook
16.17 PCI Pharma Services
16.17.1 Company Snapshot
16.17.2 Company Overview
16.17.3 Product Benchmarking
16.17.4 Strategic Outlook
16.18 Ancillare
16.18.1 Company Snapshot
16.18.2 Company Overview
16.18.3 Product Benchmarking
16.18.4 Strategic Outlook
16.19 N-SIDE
16.19.1 Company Snapshot
16.19.2 Company Overview
16.19.3 Product Benchmarking
16.19.4 Strategic Outlook
16.20 Rubicon Research Pvt S.A.
16.20.1 Company Snapshot
16.20.2 Company Overview
16.20.3 Product Benchmarking
16.20.4 Strategic Outlook
16.21 Myonex
16.21.1 Company Snapshot
16.21.2 Company Overview
16.21.3 Product Benchmarking
16.21.4 Strategic Outlook
16.22 Endpoint Clinical
16.22.1 Company Snapshot
16.22.2 Company Overview
16.22.3 Product Benchmarking
16.22.4 Strategic Outlook
16.23 Recipharm AB
16.23.1 Company Snapshot
16.23.2 Company Overview
16.23.3 Product Benchmarking
16.23.4 Strategic Outlook
16.24 COREX Logistics
16.24.1 Company Snapshot
16.24.2 Company Overview
16.24.3 Product Benchmarking
16.25 Movianto
16.25.1 Company Snapshot
16.25.2 Company Overview
16.25.3 Product Benchmarking
16.26 Durbin
16.26.1 Company Snapshot
16.26.2 Company Overview
16.26.3 Product Benchmarking
16.27 SIRO Clinpharm
16.27.1 Company Snapshot
16.27.2 Company Overview
16.27.3 Product Benchmarking
16.28 Inizio (UDG Healthcare plc)
16.28.1 Company Snapshot
16.28.2 Company Overview
16.28.3 Product Benchmarking
16.29 KLIFO
16.29.1 Company Snapshot
16.29.2 Company Overview
16.29.3 Product Benchmarking
16.30 ADAllen Pharma
16.30.1 Company Snapshot
16.30.2 Company Overview
16.30.3 Product Benchmarking
16.31 Seveillar Clinical Supplies Services
16.31.1 Company Snapshot
16.31.2 Company Overview
16.31.3 Product Benchmarking
16.32 Coghlan Group
16.32.1 Company Snapshot
16.32.2 Company Overview
16.32.3 Product Benchmarking
16.32.4 Strategic Outlook
17 Conclusion and Recommendations
17.1 Concluding Remarks from Visiongain
17.2 Recommendations for Market Players
17.2.1 Know Your Contract
17.2.2 Establish Clear Roles And Responsibilities
17.2.3 Create A Symbiotic Relationship
List of Tables
Table 1 Clinical Trial Supplies Market Snapshot, 2023 & 2033 (US$ million, CAGR %)
Table 2 Global Clinical Trial Supplies Market Forecast by Location, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 3 Offshore Clinical Site Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 4 Domestic Clinical Site Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 5 Global Clinical Trial Supplies Market Forecast by Type, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 6 Small Molecule Products Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR %)
Table 7 Biologic Products Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 8 Medical Devices Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR %)
Table 9 Global Clinical Trial Supplies Market Forecast by Services, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 10 Manufacturing Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR %)
Table 11 IMPs and INDs Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 12 Placebos Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 13 Assays and Testkits Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 14 Others Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 15 Logistics & Distribution Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 16 Cold Chain Distribution Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 17 Non-cold Chain Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 18 Storage & Retention Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 19 Packaging and Labeling Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 20 Comparator Sourcing Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 21 Other CTS Services Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 22 Global Clinical Trial Supplies Market Forecast by Therapeutic Areas, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 23 Oncology Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 24 CNS and Mental Disorders Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 25 Cardiovascular Disease (CVD) Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 26 Infectious Disease Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 27 Immunology Disease Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 28 Blood Disorders Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR %)
Table 29 Metabolic Disorders Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 30 Digestive Disorders Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 31 Other Therapeutic Areas Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR %)
Table 32 Global Clinical Trial Supplies Market Forecast by Phase, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 33 Phase-I Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 34 Phase-II Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 35 Phase-III Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 36 Phase-IV Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 37 Global Clinical Trial Supplies Market Forecast by End-User, 2023-2033 (US$ Mn, AGR%, CAGR %)
Table 38 Contract Research Organizations Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 39 Pharmaceutical and Biotechnology Companies Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 40 Medical Device Companies Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR %)
Table 41 Other End-Users Segment Market Forecast by Region, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 42 Global Clinical Trial Supplies Market Forecast by Region 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 43 North America Clinical Trial Supplies Market Forecast by Country, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 44 North America Clinical Trial Supplies Market Forecast by Location, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 45 North America Clinical Trial Supplies Market Forecast by Type, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 46 North America Clinical Trial Supplies Market Forecast by Services, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 47 North America Clinical Trial Supplies Market Forecast by Manufacturing, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 48 North America Clinical Trial Supplies Market Forecast by Logistics & Distribution, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 49 North America Clinical Trial Supplies Market Forecast by Therapeutic Areas, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 50 North America Clinical Trial Supplies Market Forecast by Phase, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 51 North America Clinical Trial Supplies Market Forecast by End-User, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 52 U.S. Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR %)
Table 53 Canada Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 54 Europe Clinical Trial Supplies Market Forecast by Country, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 55 Europe Clinical Trial Supplies Market Forecast by Location, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 56 Europe Clinical Trial Supplies Market Forecast by Type, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 57 Europe Clinical Trial Supplies Market Forecast by Services, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 58 Europe Clinical Trial Supplies Market Forecast by Manufacturing, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 59 Europe Clinical Trial Supplies Market Forecast by Logistics & Distribution, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 60 Europe Clinical Trial Supplies Market Forecast by Therapeutic Areas, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 61 Europe Clinical Trial Supplies Market Forecast by Phase, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 62 Europe Clinical Trial Supplies Market Forecast by End-User, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 63 Germany Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 64 UK Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 65 France Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 66 Italy Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 67 Spain Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 68 Russia Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 69 Rest of Europe Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 70 Asia Pacific Clinical Trial Supplies Market Forecast by Country, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 71 Asia Pacific Clinical Trial Supplies Market Forecast by Location, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 72 Asia Pacific Clinical Trial Supplies Market Forecast by Type, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 73 Asia Pacific Clinical Trial Supplies Market Forecast by Services, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 74 Asia Pacific Clinical Trial Supplies Market Forecast by Manufacturing, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 75 Asia Pacific Clinical Trial Supplies Market Forecast by Logistics & Distribution, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 76 Asia Pacific Clinical Trial Supplies Market Forecast by Therapeutic Areas, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 77 Asia Pacific Clinical Trial Supplies Market Forecast by Phase, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 78 Asia Pacific Clinical Trial Supplies Market Forecast by End-User, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 79 China Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 80 India Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 81 Japan Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 82 South Korea Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 83 Australia Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 84 Rest of Asia-Pacific Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 85 Latin America Clinical Trial Supplies Market Forecast by Country, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 86 Latin America Clinical Trial Supplies Market Forecast by Location, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 87 Latin America Clinical Trial Supplies Market Forecast by Type, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 88 Latin America Clinical Trial Supplies Market Forecast by Services, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 89 Latin America Clinical Trial Supplies Market Forecast by Manufacturing, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 90 Latin America Clinical Trial Supplies Market Forecast by Logistics & Distribution, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 91 Latin America Clinical Trial Supplies Market Forecast by Therapeutic Areas, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 92 Latin America Clinical Trial Supplies Market Forecast by Phase, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 93 Latin America Clinical Trial Supplies Market Forecast by End-User, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 94 Brazil Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 95 Mexico Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 96 Rest of Latin America Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 97 MEA Clinical Trial Supplies Market Forecast by Country, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 98 MEA Clinical Trial Supplies Market Forecast by Location, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 99 MEA Clinical Trial Supplies Market Forecast by Type, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 100 MEA Clinical Trial Supplies Market Forecast by Services, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 101 MEA Clinical Trial Supplies Market Forecast by Manufacturing, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 102 MEA Clinical Trial Supplies Market Forecast by Logistics & Distribution, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 103 MEA Clinical Trial Supplies Market Forecast by Therapeutic Areas, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 104 MEA Clinical Trial Supplies Market Forecast by Phase, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 105 MEA Clinical Trial Supplies Market Forecast by End-User, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 106 South Africa Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 107 Saudi Arabia Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 108 Turkey Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 109 UAE Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 110 Rest of MEA Clinical Trial Supplies Market Forecast, 2023-2033 (US$ Mn, AGR%, CAGR%)
Table 111 Strategic Outlook
Table 112 Thermo Fisher Scientific Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 113 Thermo Fisher Scientific Inc.: Product Benchmarking
Table 1 Thermo Fisher Scientific Inc.: Strategic Outlook
Table 114 Catalent, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 115 Catalent, Inc.: Product Benchmarking
Table 116 Catalent, Inc.: Strategic Outlook
Table 117 Piramal Pharma Solutions: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 118 Piramal Pharma Solutions: Product Benchmarking
Table 119 Piramal Pharma Solutions: Strategic Outlook
Table 120 Eurofins Scientific SE: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 121 Eurofins Scientific SE: Product Benchmarking
Table 122 Lonza: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 123 Lonza: Product Benchmarking
Table 124 Lonza: Strategic Outlook
Table 125 Clinigen Group plc: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 126 Clinigen Group plc: Product Benchmarking
Table 2 Clinigen Group plc: Strategic Outlook
Table 127 AmerisourceBergen Corporation: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 128 AmerisourceBergen Corporation: Product Benchmarking
Table 3 AmerisourceBergen Corporation: Strategic Outlook
Table 129 DHL: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 130 DHL: Product Benchmarking
Table 131 DHL: Strategic Outlook
Table 132 Sharp Corporation: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 133 Sharp Corporation: Product Benchmarking
Table 4 Sharp Corporation: Strategic Outlook
Table 134 IQVIA Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 135 IQVIA Inc.: Product Benchmarking
Table 136 IQVIA Inc.: Strategic Outlook
Table 137 Parexel: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 138 Parexel: Product Benchmarking
Table 139 Parexel: Strategic Outlook
Table 140 Biocair: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 141 Biocair: Product Benchmarking
Table 142 Biocair: Strategic Outlook
Table 143 Liveo Research: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 144 Liveo Research: Product Benchmarking
Table 145 Liveo Research: Strategic Outlook
Table 146 Marken (A UPS Company): Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 147 Marken (A UPS Company): Product Benchmarking
Table 148 Marken (A UPS Company): Strategic Outlook
Table 149 PCI Pharma Services: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 150 PCI Pharma Services: Product Benchmarking
Table 151 PCI Pharma Services: Strategic Outlook
Table 152 Ancillare: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 153 Ancillare: Product Benchmarking
Table 154 Ancillare: Strategic Outlook
Table 155 N-SIDE: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 156 N-SIDE: Product Benchmarking
Table 157 N-SIDE: Strategic Outlook
Table 158 Rubicon Research Pvt S.A.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 159 Rubicon Research Pvt S.A.: Product Benchmarking
Table 160 Rubicon Research Pvt S.A.: Strategic Outlook
Table 161 Myonex: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 162 Myonex: Product Benchmarking
Table 163 Myonex: Strategic Outlook
Table 164 Endpoint Clinical: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 165 Endpoint Clinical: Product Benchmarking
Table 166 Endpoint Clinical: Strategic Outlook
Table 167 Recipharm AB: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 168 Recipharm AB: Product Benchmarking
Table 5 Recipharm AB: Strategic Outlook
Table 169 COREX Logistics: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 170 COREX Logistics: Product Benchmarking
Table 171 Movianto: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 172 Movianto: Product Benchmarking
Table 173 Durbin: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 174 Durbin: Product Benchmarking
Table 175 SIRO Clinpharm: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 176 SIRO Clinpharm: Product Benchmarking
Table 177 Inizio (UDG Healthcare plc): Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 178 Inizio (UDG Healthcare plc): Product Benchmarking
Table 179 KLIFO: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 180 KLIFO: Product Benchmarking
Table 181 ADAllen Pharma: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 182 ADAllen Pharma: Product Benchmarking
Table 183 Seveillar Clinical Supplies Services: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 184 Seveillar Clinical Supplies Services: Product Benchmarking
Table 185 Coghlan Group: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 186 Coghlan Group: Product Benchmarking
Table 187 Coghlan Group: Strategic Outlook
List of Figures
Figure 1 Clinical Trial Supplies Market Segmentation
Figure 2 Clinical Trial Supplies Market by Location: Market Attractiveness Index
Figure 3 Clinical Trial Supplies Market by Type: Market Attractiveness Index
Figure 4 Clinical Trial Supplies Market by Services: Market Attractiveness Index
Figure 5 Clinical Trial Supplies Market by Therapeutic Areas: Market Attractiveness Index
Figure 6 Clinical Trial Supplies Market by Phase: Market Attractiveness Index
Figure 7 Clinical Trial Supplies Market by End-User: Market Attractiveness Index
Figure 8 Clinical Trial Supplies Market Attractiveness Index by Region
Figure 9 Clinical Trial Supplies Market: Market Dynamics
Figure 10 COVID Impact Analysis: Clinical Trial Supplies Market Recovery Scenarios
Figure 11 Clinical Trial Supplies Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “V” Shaped Recovery Scenario
Figure 12 Clinical Trial Supplies Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “V” Shaped Recovery
Figure 13 Clinical Trial Supplies Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “U” Shaped Recovery Scenario
Figure 14 Clinical Trial Supplies Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “U” Shaped Recovery
Figure 15 Clinical Trial Supplies Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “W” Shaped Recovery Scenario
Figure 16 Clinical Trial Supplies Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “W” Shaped Recovery
Figure 17 Clinical Trial Supplies Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “L” Shaped Recovery Scenario
Figure 18 Clinical Trial Supplies Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “L” Shaped Recovery
Figure 19 Clinical Trial Supplies Market: PEST Analysis
Figure 20 Clinical Trial Supplies Market by Location: Market Attractiveness Index
Figure 21 Clinical Trial Supplies Market Forecast by Location, 2023-2033 (US$ million)
Figure 22 Clinical Trial Supplies Market Share Forecast by Location, 2023, 2028, 2033 (%)
Figure 23 Offshore Clinical Site Market Forecast by Region, 2023-2033 (US$ million)
Figure 24 Offshore Clinical Site Market Share Forecast by Region, 2023 & 2033 (%)
Figure 25 Offshore Clinical Site Market Forecast by Region, 2023-2033 (US$ million)
Figure 26 Offshore Clinical Site Market Share Forecast by Region, 2023 & 2033 (%)
Figure 27 Clinical Trial Supplies Market by Type: Market Attractiveness Index
Figure 28 Clinical Trial Supplies Market Forecast by Type, 2023-2033 (US$ million)
Figure 29 Clinical Trial Supplies Market Share Forecast by Type, 2023, 2028, 2033 (%)
Figure 30 Small Molecule Products Market Forecast by Region, 2023-2033 (US$ million)
Figure 31 Small Molecule Products Market Share Forecast by Region, 2023 & 2033 (%)
Figure 32 Biologic Products Market Forecast by Region, 2023-2033 (US$ million)
Figure 33 Biologic Products Market Share Forecast by Region, 2023 & 2033 (%)
Figure 34 Medical Devices Market Forecast by Region, 2023-2033 (US$ million)
Figure 35 Medical Devices Market Share Forecast by Region, 2023 & 2033 (%)
Figure 36 Clinical Trial Supplies Market by Services: Market Attractiveness Index
Figure 37 Clinical Trial Supplies Market Forecast by Services, 2023-2033 (US$ million)
Figure 38 Clinical Trial Supplies Market Share Forecast by Services, 2023, 2028, 2033 (%)
Figure 39 Manufacturing Market Forecast by Region, 2023-2033 (US$ million)
Figure 40 Manufacturing Market Share Forecast by Region, 2023 & 2033 (%)
Figure 41 IMPs and INDs Market Forecast by Region, 2023-2033 (US$ million)
Figure 42 IMPs and INDs Market Share Forecast by Region, 2023 & 2033 (%)
Figure 43 Placebos Market Forecast by Region, 2023-2033 (US$ million)
Figure 44 Placebos Market Share Forecast by Region, 2023 & 2033 (%)
Figure 45 Assays and Testkits Market Forecast by Region, 2023-2033 (US$ million)
Figure 46 Assays and Testkits Market Share Forecast by Region, 2023 & 2033 (%)
Figure 47 Others Market Forecast by Region, 2023-2033 (US$ million)
Figure 48 Others Market Share Forecast by Region, 2023 & 2033 (%)
Figure 49 Logistics & Distribution Market Forecast by Region, 2023-2033 (US$ million)
Figure 50 Logistics & Distribution Market Share Forecast by Region, 2023 & 2033 (%)
Figure 51 Cold Chain Distribution Market Forecast by Region, 2023-2033 (US$ million)
Figure 52 Cold Chain Distribution Market Share Forecast by Region, 2023 & 2033 (%)
Figure 53 Non-cold Chain Market Forecast by Region, 2023-2033 (US$ million)
Figure 54 Non-cold Chain Market Share Forecast by Region, 2023 & 2033 (%)
Figure 55 Logistics & Distribution Market Forecast by Region, 2023-2033 (US$ million)
Figure 56 Storage & Retention Market Share Forecast by Region, 2023 & 2033 (%)
Figure 57 Packaging and Labeling Market Forecast by Region, 2023-2033 (US$ million)
Figure 58 Packaging and Labeling Market Share Forecast by Region, 2023 & 2033 (%)
Figure 59 Comparator Sourcing Market Forecast by Region, 2023-2033 (US$ million)
Figure 60 Comparator Sourcing Market Share Forecast by Region, 2023 & 2033 (%)
Figure 61 Other CTS Services Market Forecast by Region, 2023-2033 (US$ million)
Figure 62 Other CTS Services Market Share Forecast by Region, 2023 & 2033 (%)
Figure 63 Clinical Trial Supplies Market by Therapeutic Areas: Market Attractiveness Index
Figure 64 Clinical Trial Supplies Market Forecast by Therapeutic Areas, 2023-2033 (US$ million)
Figure 65 Clinical Trial Supplies Market Share Forecast by Therapeutic Areas, 2023, 2028, 2033 (%)
Figure 66 Oncology Market Forecast by Region, 2023-2033 (US$ million)
Figure 67 Oncology Market Share Forecast by Region, 2023 & 2033 (%)
Figure 68 CNS and Mental Disorders Market Forecast by Region, 2023-2033 (US$ million)
Figure 69 CNS and Mental Disorders Market Share Forecast by Region, 2023 & 2033 (%)
Figure 70 Cardiovascular Disease (CVD) Market Forecast by Region, 2023-2033 (US$ million)
Figure 71 Cardiovascular Disease (CVD) Market Share Forecast by Region, 2023 & 2033 (%)
Figure 72 Cardiovascular Disease (CVD) Market Forecast by Region, 2023-2033 (US$ million)
Figure 73 Cardiovascular Disease (CVD) Market Share Forecast by Region, 2023 & 2033 (%)
Figure 74 Oncology Market Forecast by Region, 2023-2033 (US$ million)
Figure 75 Oncology Market Share Forecast by Region, 2023 & 2033 (%)
Figure 76 Blood Disorders Market Forecast by Region, 2023-2033 (US$ million)
Figure 77 Blood Disorders Market Share Forecast by Region, 2023 & 2033 (%)
Figure 78 Metabolic Disorders Market Forecast by Region, 2023-2033 (US$ million)
Figure 79 Metabolic Disorders Market Share Forecast by Region, 2023 & 2033 (%)
Figure 80 Metabolic Disorders Market Forecast by Region, 2023-2033 (US$ million)
Figure 81 Digestive Disorders Market Share Forecast by Region, 2023 & 2033 (%)
Figure 82 Other Therapeutic Areas Market Forecast by Region, 2023-2033 (US$ million)
Figure 83 Other Therapeutic Areas Market Share Forecast by Region, 2023 & 2033 (%)
Figure 84 Clinical Trial Supplies Market by Phase: Market Attractiveness Index
Figure 85 Clinical Trial Supplies Market Forecast by Phase, 2023-2033 (US$ million)
Figure 86 Clinical Trial Supplies Market Share Forecast by Phase, 2023, 2028, 2033 (%)
Figure 87 Phase-I Market Forecast by Region, 2023-2033 (US$ million)
Figure 88 Phase-I Market Share Forecast by Region, 2023 & 2033 (%)
Figure 89 Phase-II Market Forecast by Region, 2023-2033 (US$ million)
Figure 90 Phase-II Market Share Forecast by Region, 2023 & 2033 (%)
Figure 91 Phase-III Market Forecast by Region, 2023-2033 (US$ million)
Figure 92 Phase-III Market Share Forecast by Region, 2023 & 2033 (%)
Figure 93 Phase-IV Market Forecast by Region, 2023-2033 (US$ million)
Figure 94 Phase-IV Market Share Forecast by Region, 2023 & 2033 (%)
Figure 95 Clinical Trial Supplies Market by End-User: Market Attractiveness Index
Figure 96 Clinical Trial Supplies Market Forecast by End-User, 2023-2033 (US$ million)
Figure 97 Clinical Trial Supplies Market Share Forecast by End-User, 2023, 2028, 2033 (%)
Figure 98 Phase-I Market Forecast by Region, 2023-2033 (US$ million)
Figure 99 Phase-I Market Share Forecast by Region, 2023 & 2033 (%)
Figure 100 Pharmaceutical and Biotechnology Companies Market Forecast by Region, 2023-2033 (US$ million)
Figure 101 Pharmaceutical and Biotechnology Companies Market Share Forecast by Region, 2023 & 2033 (%)
Figure 102 Medical Device Companies Market Forecast by Region, 2023-2033 (US$ million)
Figure 103 Medical Device Companies Market Share Forecast by Region, 2023 & 2033 (%)
Figure 104 Medical Device Companies Market Forecast by Region, 2023-2033 (US$ million)
Figure 105 Other End-Users Market Share Forecast by Region, 2023 & 2033 (%)
Figure 106 Clinical Trial Supplies Market Forecast by Region 2023, 2028, 2033 (Revenue, CAGR%)
Figure 107 Clinical Trial Supplies Market Share Forecast by Region 2023, 2028, 2033(%)
Figure 108 Clinical Trial Supplies Market by Region, 2023-2033 (US$ Mn)
Figure 109 North America Clinical Trial Supplies Market Attractiveness Index
Figure 110 North America Clinical Trial Supplies Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 111 North America Clinical Trial Supplies Market Forecast by Country, 2023-2033 (US$ million)
Figure 112 North America Clinical Trial Supplies Market Share Forecast by Country, 2023 & 2033 (%)
Figure 113 North America Clinical Trial Supplies Market Forecast by Location, 2023-2033 (US$ million)
Figure 114 North America Clinical Trial Supplies Market Share Forecast by Location, 2023 & 2033 (%)
Figure 115 North America Clinical Trial Supplies Market Forecast by Type, 2023-2033 (US$ million)
Figure 116 North America Clinical Trial Supplies Market Share Forecast by Type, 2023 & 2033 (%)
Figure 117 North America Clinical Trial Supplies Market Forecast by Services, 2023-2033 (US$ million)
Figure 118 North America Clinical Trial Supplies Market Share Forecast by Services, 2023 & 2033 (%)
Figure 119 North America Clinical Trial Supplies Market Forecast by Manufacturing, 2023-2033 (US$ million)
Figure 120 North America Clinical Trial Supplies Market Share Forecast by Manufacturing, 2023 & 2033 (%)
Figure 121 North America Clinical Trial Supplies Market Forecast by Logistics & Distribution, 2023-2033 (US$ million)
Figure 122 North America Clinical Trial Supplies Market Share Forecast by Logistics & Distribution, 2023 & 2033 (%)
Figure 123 North America Clinical Trial Supplies Market Forecast by Therapeutic Areas, 2023-2033 (US$ million)
Figure 124 North America Clinical Trial Supplies Market Share Forecast by Therapeutic Areas, 2023 & 2033 (%)
Figure 125 North America Clinical Trial Supplies Market Forecast by Phase, 2023-2033 (US$ million)
Figure 126 North America Clinical Trial Supplies Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 127 North America Clinical Trial Supplies Market Forecast by End-User, 2023-2033 (US$ million)
Figure 128 North America Clinical Trial Supplies Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 129 U.S. Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 130 Canada Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 131 Europe Clinical Trial Supplies Market Attractiveness Index
Figure 132 Europe Clinical Trial Supplies Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 133 Europe Clinical Trial Supplies Market Forecast by Country, 2023-2033 (US$ million)
Figure 134 Europe Clinical Trial Supplies Market Share Forecast by Country, 2023 & 2033 (%)
Figure 135 Europe Clinical Trial Supplies Market Forecast by Location, 2023-2033 (US$ million)
Figure 136 Europe Clinical Trial Supplies Market Share Forecast by Location, 2023 & 2033 (%)
Figure 137 Europe Clinical Trial Supplies Market Forecast by Type, 2023-2033 (US$ million)
Figure 138 Europe Clinical Trial Supplies Market Share Forecast by Type, 2023 & 2033 (%)
Figure 139 Europe Clinical Trial Supplies Market Forecast by Services, 2023-2033 (US$ million)
Figure 140 Europe Clinical Trial Supplies Market Share Forecast by Services, 2023 & 2033 (%)
Figure 141 Europe Clinical Trial Supplies Market Forecast by Manufacturing, 2023-2033 (US$ million)
Figure 142 Europe Clinical Trial Supplies Market Share Forecast by Manufacturing, 2023 & 2033 (%)
Figure 143 Europe Clinical Trial Supplies Market Forecast by Logistics & Distribution, 2023-2033 (US$ million)
Figure 144 Europe Clinical Trial Supplies Market Share Forecast by Logistics & Distribution, 2023 & 2033 (%)
Figure 145 Europe Clinical Trial Supplies Market Forecast by Therapeutic Areas, 2023-2033 (US$ million)
Figure 146 Europe Clinical Trial Supplies Market Share Forecast by Therapeutic Areas, 2023 & 2033 (%)
Figure 147 Europe Clinical Trial Supplies Market Forecast by Phase, 2023-2033 (US$ million)
Figure 148 Europe Clinical Trial Supplies Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 149 Europe Clinical Trial Supplies Market Forecast by End-User, 2023-2033 (US$ million)
Figure 150 Europe Clinical Trial Supplies Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 151 Germany Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 152 UK Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 153 France Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 154 Italy Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 155 Spain Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 156 Russia Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 157 Rest of Europe Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 158 Asia Pacific Clinical Trial Supplies Market Attractiveness Index
Figure 159 Asia Pacific Clinical Trial Supplies Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 160 Asia Pacific Clinical Trial Supplies Market Forecast by Country, 2023-2033 (US$ million)
Figure 161 Asia Pacific Clinical Trial Supplies Market Share Forecast by Country, 2023 & 2033 (%)
Figure 162 Asia Pacific Clinical Trial Supplies Market Forecast by Location, 2023-2033 (US$ million)
Figure 163 Asia Pacific Clinical Trial Supplies Market Share Forecast by Location, 2023 & 2033 (%)
Figure 164 Asia Pacific Clinical Trial Supplies Market Forecast by Type, 2023-2033 (US$ million)
Figure 165 Asia Pacific Clinical Trial Supplies Market Share Forecast by Type, 2023 & 2033 (%)
Figure 166 Asia Pacific Clinical Trial Supplies Market Forecast by Services, 2023-2033 (US$ million)
Figure 167 Asia Pacific Clinical Trial Supplies Market Share Forecast by Services, 2023 & 2033 (%)
Figure 168 Asia Pacific Clinical Trial Supplies Market Forecast by Manufacturing, 2023-2033 (US$ million)
Figure 169 Asia Pacific Clinical Trial Supplies Market Share Forecast by Manufacturing, 2023 & 2033 (%)
Figure 170 Asia Pacific Clinical Trial Supplies Market Forecast by Logistics & Distribution, 2023-2033 (US$ million)
Figure 171 Asia Pacific Clinical Trial Supplies Market Share Forecast by Logistics & Distribution, 2023 & 2033 (%)
Figure 172 Asia Pacific Clinical Trial Supplies Market Forecast by Therapeutic Areas, 2023-2033 (US$ million)
Figure 173 Asia Pacific Clinical Trial Supplies Market Share Forecast by Therapeutic Areas, 2023 & 2033 (%)
Figure 174 Asia Pacific Clinical Trial Supplies Market Forecast by Phase, 2023-2033 (US$ million)
Figure 175 Asia Pacific Clinical Trial Supplies Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 176 Asia Pacific Clinical Trial Supplies Market Forecast by End-User, 2023-2033 (US$ million)
Figure 177 Asia Pacific Clinical Trial Supplies Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 178 China Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 179 India Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 180 Japan Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 181 South Korea Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 182 Australia Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 183 Rest of Asia-Pacific Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 184 Latin America Clinical Trial Supplies Market Attractiveness Index
Figure 185 Latin America Clinical Trial Supplies Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 186 Latin America Clinical Trial Supplies Market Forecast by Country, 2023-2033 (US$ million)
Figure 187 Latin America Clinical Trial Supplies Market Share Forecast by Country, 2023 & 2033 (%)
Figure 188 Latin America Clinical Trial Supplies Market Forecast by Location, 2023-2033 (US$ million)
Figure 189 Latin America Clinical Trial Supplies Market Share Forecast by Location, 2023 & 2033 (%)
Figure 190 Latin America Clinical Trial Supplies Market Forecast by Type, 2023-2033 (US$ million)
Figure 191 Latin America Clinical Trial Supplies Market Share Forecast by Type, 2023 & 2033 (%)
Figure 192 Latin America Clinical Trial Supplies Market Forecast by Services, 2023-2033 (US$ million)
Figure 193 Latin America Clinical Trial Supplies Market Share Forecast by Services, 2023 & 2033 (%)
Figure 194 Latin America Clinical Trial Supplies Market Forecast by Manufacturing, 2023-2033 (US$ million)
Figure 195 Latin America Clinical Trial Supplies Market Share Forecast by Manufacturing, 2023 & 2033 (%)
Figure 196 Latin America Clinical Trial Supplies Market Forecast by Logistics & Distribution, 2023-2033 (US$ million)
Figure 197 Latin America Clinical Trial Supplies Market Share Forecast by Logistics & Distribution, 2023 & 2033 (%)
Figure 198 Latin America Clinical Trial Supplies Market Forecast by Therapeutic Areas, 2023-2033 (US$ million)
Figure 199 Latin America Clinical Trial Supplies Market Share Forecast by Therapeutic Areas, 2023 & 2033 (%)
Figure 200 Latin America Clinical Trial Supplies Market Forecast by Phase, 2023-2033 (US$ million)
Figure 201 Latin America Clinical Trial Supplies Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 202 Latin America Clinical Trial Supplies Market Forecast by End-User, 2023-2033 (US$ million)
Figure 203 Latin America Clinical Trial Supplies Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 204 Brazil Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 205 Mexico Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 206 Rest of Latin America Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 207 MEA Clinical Trial Supplies Market Attractiveness Index
Figure 208 MEA Clinical Trial Supplies Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 209 MEA Clinical Trial Supplies Market Forecast by Country, 2023-2033 (US$ million)
Figure 210 MEA Clinical Trial Supplies Market Share Forecast by Country, 2023 & 2033 (%)
Figure 211 MEA Clinical Trial Supplies Market Forecast by Location, 2023-2033 (US$ million)
Figure 212 MEA Clinical Trial Supplies Market Share Forecast by Location, 2023 & 2033 (%)
Figure 213 MEA Clinical Trial Supplies Market Forecast by Type, 2023-2033 (US$ million)
Figure 214 MEA Clinical Trial Supplies Market Share Forecast by Type, 2023 & 2033 (%)
Figure 215 MEA Clinical Trial Supplies Market Forecast by Services, 2023-2033 (US$ million)
Figure 216 MEA Clinical Trial Supplies Market Share Forecast by Services, 2023 & 2033 (%)
Figure 217 MEA Clinical Trial Supplies Market Forecast by Manufacturing, 2023-2033 (US$ million)
Figure 218 MEA Clinical Trial Supplies Market Share Forecast by Manufacturing, 2023 & 2033 (%)
Figure 219 MEA Clinical Trial Supplies Market Forecast by Logistics & Distribution, 2023-2033 (US$ million)
Figure 220 MEA Clinical Trial Supplies Market Share Forecast by Logistics & Distribution, 2023 & 2033 (%)
Figure 221 MEA Clinical Trial Supplies Market Forecast by Therapeutic Areas, 2023-2033 (US$ million)
Figure 222 MEA Clinical Trial Supplies Market Share Forecast by Therapeutic Areas, 2023 & 2033 (%)
Figure 223 MEA Clinical Trial Supplies Market Forecast by Phase, 2023-2033 (US$ million)
Figure 224 MEA Clinical Trial Supplies Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 225 MEA Clinical Trial Supplies Market Forecast by End-User, 2023-2033 (US$ million)
Figure 226 MEA Clinical Trial Supplies Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 227 South Africa Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 228 Saudi Arabia Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 229 Turkey Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 230 UAE Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
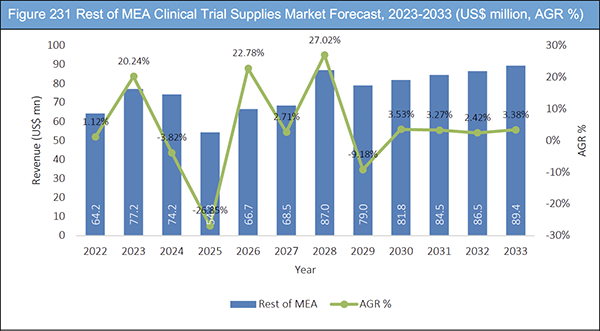
Figure 231 Rest of MEA Clinical Trial Supplies Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 232 Clinical Trial Supplies Market: Company Share, 2021
Figure 233 Thermo Fisher Scientific Inc.: Net Revenue, 2017-2021 (US$ million, AGR%)
Figure 234 Thermo Fisher Scientific Inc.: R&D, 2017-2021 (US$ million, AGR%)
Figure 235 Thermo Fisher Scientific Inc.: Gross Profit, 2017-2021 (US$ million, AGR%)
Figure 236 Thermo Fisher Scientific Inc.: Regional Market Shares, 2021
Figure 237 Catalent, Inc.: Net Revenue, 2017-2021 (US$ million, AGR%)
Figure 238 Catalent, Inc.: EBITDA, 2017-2021 (US$ million, AGR%)
Figure 239 Catalent, Inc.: Net Income, 2017-2021 (US$ million, AGR%)
Figure 240 Catalent, Inc.: Regional Market Shares, 2021
Figure 241 Eurofins Scientific SE: Net Revenue, 2017-2021 (US$ million, AGR%)
Figure 242 Eurofins Scientific SE: Operating Income, 2017-2021 (US$ million, AGR%)
Figure 243 Eurofins Scientific SE: EBITDA, 2017-2021 (US$ million, AGR%)
Figure 244 Eurofins Scientific SE: Net Income, 2017-2021 (US$ million, AGR%)
Figure 245 Eurofins Scientific SE: Regional Market Shares, 2021
Figure 246 Lonza: Net Revenue, 2017-2021 (US$ million, AGR%)
Figure 247 Lonza: EBIT, 2017-2021 (US$ million, AGR%)
Figure 248 Lonza: Gross Profit, 2017-2021 (US$ million, AGR%)
Figure 249 Clinigen Group plc: Net Revenue, 2017-2021 (US$ million, AGR%)
Figure 250 Clinigen Group plc: EBITDA, 2017-2021 (US$ million, AGR%)
Figure 251 Clinigen Group plc: Net Income, 2017-2021 (US$ million, AGR%)
Figure 252 AmerisourceBergen Corporation: Net Revenue, 2017-2021 (US$ million, AGR%)
Figure 253 AmerisourceBergen Corporation: Operating Income, 2017-2021 (US$ million, AGR%)
Figure 254 AmerisourceBergen Corporation: EBITDA, 2017-2021 (US$ million, AGR%)
Figure 255 AmerisourceBergen Corporation: Net Income, 2017-2021 (US$ million, AGR%)
Figure 256 DHL: Net Revenue, 2017-2021 (US$ million, AGR%)
Figure 257 DHL: EBIT, 2017-2021 (US$ million, AGR%)
Figure 258 DHL: Gross Profit, 2017-2021 (US$ million, AGR%)
Figure 259 DHL: Net Income Profit, 2017-2021 (US$ million, AGR %)
Figure 260 DHL: Operating Income, 2017-2021 (US$ million, AGR%)
Figure 261 DHL: Regional Market Share, 2017-2021 (US$ million, AGR%)
Figure 262 Sharp Corporation: Net Revenue, 2017-2021 (US$ million, AGR%)
Figure 263 Sharp Corporation: EBITDA, 2017-2021 (US$ million, AGR%)
Figure 264 Sharp Corporation: Net Income, 2017-2021 (US$ million, AGR%)
Figure 265 Sharp Corporation: Operating Income, 2017-2021 (US$ million, AGR%)
Figure 266 Sharp Corporation: Gross Profit, 2017-2021 (US$ million, AGR%)
Figure 267 IQVIA Inc.: Net Revenue, 2017-2021 (US$ million, AGR%)
Figure 268 IQVIA Inc.: EBIT, 2017-2021 (US$ million, AGR%)
Figure 269 IQVIA Inc.: Gross Profit, 2017-2021 (US$ million, AGR%)
Figure 270 IQVIA Inc.: Net Income Profit, 2017-2021 (US$ million, AGR%)
Figure 271 IQVIA Inc.: Operating Income, 2017-2021 (US$ million, AGR%)
Figure 272 IQVIA Inc.: Regional Market Share, 2017-2021 (US$ million, AGR%)
Table 1 Global Women’s Health Market Snapshot, 2023 & 2033 (US$ million, CAGR %)
Table 2 Women's Health Market, By Region, 2022-2032 (USD Mn, AGR %, CAGR %) (V-shaped Recovery)
Table 3 Women's Health Market, By Region, 2022-2032 (USD Mn, AGR %, CAGR %) (U-shaped Recovery)
Table 4 Women's Health Market, By Region, 2022-2032 (USD Mn, AGR %, CAGR %) (W-shaped Recovery)
Table 5 Women's Health Market, By Region, 2022-2032 (USD Mn, AGR %, CAGR %) (L-shaped Recovery)
Table 6 Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 7 Contraceptives Market, by Region, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 8 Postmenopausal Osteoporosis Market, by Region, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 9 Hormonal Infertility Market, by Region, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 10 Menopause Market, by Region, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 11 Polycystic Ovary Syndrome Market, by Region, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 12 Endometriosis and Uterine Fibroids Market, by Region, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 13 Other Indications Market, by Region, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 14 Women's Health Distribution Channel Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 15 Hospital Pharmacies Market, by Region, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 16 Retail Pharmacies Market, by Region, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 17 Online Pharmacies Market, by Region, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 18 Xgeva Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 19 Prolia Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 20 Mirena Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 21 Forteo Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 22 Premarin Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 23 Lupron Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 24 Lo Loestrin Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 25 Orilissa/Oriahnn Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 26 Generics Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 27 Branded Drugs Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 28 Women's Health Market, By Region, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 29 Women's Health Market, By Region, 2022-2032 (USD Mn, AGR %, CAGR %) (V-shaped Recovery)
Table 30 Women's Health Market, By Region, 2022-2032 (USD Mn, AGR %, CAGR %) (U-shaped Recovery)
Table 31 Women's Health Market, By Region, 2022-2032 (USD Mn, AGR %, CAGR %) (W-shaped Recovery)
Table 32 Women's Health Market, By Region, 2022-2032 (USD Mn, AGR %, CAGR %) (L-shaped Recovery)
Table 33 North America Women's Health Market, By Country, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 34 North America Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 35 North America Gynecology Distribution Channel Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 36 US Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 37 US Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 38 Canada Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 39 Canada Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 40 Europe Women's Health Market, By Country, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 41 Europe Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 42 Europe Gynecology Distribution Channel Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 43 Germany Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 44 Germany Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 45 UK Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 46 UK Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 47 France Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 48 France Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 49 Italy Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 50 Italy Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 51 Spain Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 52 Spain Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 53 Rest of Europe Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 54 Rest of Europe Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 55 Asia Pacific Women's Health Market, By Country, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 56 Asia Pacific Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 57 Asia-Pacific Gynecology Distribution Channel Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 58 Japan Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 59 Japan Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 60 China Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 61 China Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 62 India Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 63 India Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 64 South Korea Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 65 South Korea Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 66 Australia Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 67 Australia Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 68 Rest of Asia Pacific Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 69 Rest of Asia Pacific Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 70 Latin America Women's Health Market, By Country, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 71 Latin America Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 72 Latin America Gynecology Distribution Channel Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 73 Brazil Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 74 Brazil Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 75 Latin America Women's Health Market, By Country, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 76 Mexico Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 77 Rest of Latin America Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 78 Rest of LATAM Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 79 MEA Women's Health Market, By Country, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 80 MEA Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 81 MEA Gynecology Distribution Channel Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 82 South Africa Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 83 South Africa Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 84 GCC Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 85 GCC Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 86 Rest of MEA Women's Health Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 87 Rest of MEA Women's Health Disease Market, 2023-2033 (USD Mn, AGR (%), CAGR (%))
Table 88 Key Business Strategies Adopted by Key Players in Global Women’s Health Market
Table 89 Pfizer: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 90 Pfizer: Product Benchmarking
Table 91 Pfizer Inc: Company Product Pipeline
Table 92 Pfizer Inc: Company Recent Developments
Table 93 Merck: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 94 Merck: Product Benchmarking
Table 95 Merck: Company Recent Developments
Table 96 Bayer Pharmaceuticals: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 97 Bayer Pharmaceuticals: Product Benchmarking
Table 98 Bayer Pharmaceuticals Inc: Company Product Pipeline
Table 99 Bayer Pharmaceuticals: Company Recent Developments
Table 100 Eli Lilly and Company: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 101 Eli Lilly and Company: Product Benchmarking
Table 102 Amgen: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 103 Amgen: Product Benchmarking
Table 104 Amgen: Strategic Outlook
Table 105 AbbVie: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 106 AbbVie: Product Benchmarking
Table 107 AbbVie: Company Product Pipeline
Table 108 AbbVie: Company Recent Developments
Table 109 Novartis: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 110 Novartis: Product Benchmarking
Table 111 Agile Therapeutics: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 112 Agile Therapeutics: Product Benchmarking
Table 113 Agile Therapeutics: Company Product Pipeline
Table 114 Agile Therapeutics: Company Recent Developments
Table 115 Ferring B.V.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 116 Ferring B.V.: Product Benchmarking
Table 117 Ferring B.V.: Company Product Pipeline
Table 118 Ferring B.V.: Company Recent Developments
Table 119 Apothecus Pharmaceutical Corp.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 120 Apothecus Pharmaceutical Corp.: Product Benchmarking
List of Figures
Figure 1 Global Women’s Health Market Segmentation
Figure 2 Global Women’s Health Market Forecast by Region: Market Attractiveness Index
Figure 3 Global Women’s Health Market by Disease: Market Attractiveness Index
Figure 4 Global Women’s Health Market by Distribution Channel: Market Attractiveness Index
Figure 5 Global Women’s Health Market: Market Dynamics
Figure 6 Global COVID Impact Analysis: Women’s Health Market Recovery Scenarios
Figure 7 Global Women’s Health Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “V” Shaped Recovery
Figure 8 Global Women’s Health Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “U” Shaped Recovery
Figure 9 Global Women’s Health Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “W” Shaped Recovery
Figure 10 Global Women’s Health Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “L” Shaped Recovery
Figure 11 Global Women’s Health Market: Porter’s Five Forces Analysis
Figure 12 Global Women’s Health Market Forecast by Disease 2023 (Revenue, CAGR%)
Figure 13 Global Women’s Health Market Share Forecast by Disease, 2023 and 2033 (%)
Figure 14 Global Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 15 Contraceptives Market Forecast by Region, 2023-2033 (US$ million)
Figure 16 Contraceptives Market Share Forecast by Region, 2023, 2028, 2033 (%)
Figure 17 Postmenopausal Osteoporosis Market Forecast by Region, 2023-2033 (US$ million)
Figure 18 Postmenopausal Osteoporosis Market Share Forecast by Region, 2023, 2028, 2033 (%)
Figure 19 Hormonal Infertility Market Forecast by Region, 2023-2033 (US$ million)
Figure 20 Hormonal Infertility Market Share Forecast by Region, 2023-2033 (%)
Figure 21 Menopause Market Forecast by Region, 2023-2033 (US$ million)
Figure 22 Menopause Market Share Forecast by Region, 2023-2033 (%)
Figure 23 Polycystic Ovary Syndrome Market Forecast by Region, 2023-2033 (US$ million)
Figure 24 Polycystic Ovary Syndrome Market Share Forecast by Region, 2023, 2028, 2033 (%)
Figure 25 Endometriosis and Uterine Fibroids Market Forecast by Region, 2023-2033 (US$ million)
Figure 26 Endometriosis and Uterine Fibroids Market Share Forecast by Region, 2023, 2028, 2033 (%)
Figure 27 Other Disease Market Forecast by Region, 2023-2033 (US$ million)
Figure 28 Other Disease Market Share Forecast by Region, 2023, 2028, 2033 (%)
Figure 29 Global Women’s Health Market Forecast by Distribution Channel 2023 and 2033 (Revenue, CAGR%)
Figure 30 Global Women’s Health Market Forecast by Distribution Channel, 2023-2033 (US$ million, AGR %)
Figure 31 Global Women’s Health Market Share Forecast by Distribution Channel, 2023, 2033 (%)
Figure 32 Hospital Pharmacies Market Forecast by Region, 2023-2033 (US$ million)
Figure 33 Hospital Pharmacies Market Share Forecast by Region, 2023, 2028, 2033 (%)
Figure 34 Retail Pharmacies Market Forecast by Region, 2023-2033 (US$ million)
Figure 35 Retail Pharmacies Market Share Forecast by Region, 2023, 2028, 2033 (%)
Figure 36 Online Pharmacies Market Forecast by Region, 2023-2033 (US$ million)
Figure 37 Online Pharmacies Market Share Forecast by Region, 2023, 2028, 2033 (%)
Figure 38 Xgeva Market Forecast, 2023-2033 (US$ million)
Figure 39 Prolia Market Forecast, 2023-2033 (US$ million)
Figure 40 Mirena Market Forecast, 2023-2033 (US$ million)
Figure 41 Forteo Market Forecast, 2023-2033 (US$ million)
Figure 42 Premarin Market Forecast, 2023-2033 (US$ million)
Figure 43 Lupron Market Forecast, 2023-2033 (US$ million)
Figure 44 Lo Loestrin Market Forecast, 2023-2033 (US$ million)
Figure 45 Orilissa/Oriahnn Market Forecast, 2023-2033 (US$ million)
Figure 46 Global Women’s Health Market Forecast by Generics/Branded 2023 (Revenue, CAGR%)
Figure 47 Global Women’s Health Market Share Forecast by Generics/Branded, 2023, 2033 (%)
Figure 48 Global Women’s Health Market Forecast by Generics, 2023-2033 (US$ million, AGR %)
Figure 49 Global Women’s Health Market Forecast by Branded, 2023-2033 (US$ million, AGR %)
Figure 50 Global Women’s Health Market Forecast by Region 2023 and 2033 (Revenue)
Figure 51 Global Women’s Health Market Share Forecast by Region 2023 and 2033(%)
Figure 52 Global Women’s Health Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%))
Figure 53 Global Women’s Health Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “V” Shaped Recovery
Figure 54 Global Women’s Health Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “U” Shaped Recovery
Figure 55 Global Women’s Health Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “W” Shaped Recovery
Figure 56 Global Women’s Health Market by Region, 2023-2033 (US$ Mn, AGR (%), CAGR (%)): “L” Shaped Recovery
Figure 57 North America Women’s Health Market Attractiveness Index, 2023
Figure 58 North America Women’s Health Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 59 North America Women’s Health Market Forecast by Country, 2023-2033 (US$ million, AGR %)
Figure 60 North America Women’s Health Market Share Forecast by Country, 2023 & 2033 (%)
Figure 61 North America Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 62 North America Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 63 North America Women’s Health Market Forecast by Distribution Channel, 2023-2033 (US$ million, AGR %)
Figure 64 North America Women’s Health Market Share Forecast by Distribution Channel, 2023 & 2033 (%)
Figure 65 U.S. Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 66 U.S. Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 67 U.S. Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 68 Canada Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 69 Canada Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 70 Canada Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 71 Europe Women’s Health Market Attractiveness Index
Figure 72 Europe Women’s Health Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 73 Europe Women’s Health Market Forecast by Country, 2023-2033 (US$ million, AGR %)
Figure 74 Europe Women’s Health Market Share Forecast by Country, 2023 & 2033 (%)
Figure 75 Europe Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 76 Europe Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 77 Europe Women’s Health Market Forecast by Distribution Channel, 2023-2033 (US$ million, AGR %)
Figure 78 Europe Women’s Health Market Share Forecast by Distribution Channel, 2023 & 2033 (%)
Figure 79 Germany Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 80 Germany Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 81 Germany Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 82 UK Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 83 U.K. Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 84 U.K. Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 85 France Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 86 France Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 87 France Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 88 Italy Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 89 Italy Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 90 Italy Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 91 Spain Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 92 Spain Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 93 Spain Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 94 Rest of Europe Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 95 Rest of Europe Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 96 Rest of Europe Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 97 Asia Pacific Women’s Health Market Attractiveness Index, 2023
Figure 98 Asia Pacific Women’s Health Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 99 Asia Pacific Women’s Health Market Forecast by Country, 2023-2033 (US$ million, AGR %)
Figure 100 Asia Pacific Women’s Health Market Share Forecast by Country, 2023 & 2033 (%)
Figure 101 Asia Pacific Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 102 Asia Pacific Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 103 Asia Pacific Women’s Health Market Forecast by Distribution Channel, 2023-2033 (US$ million, AGR %)
Figure 104 Asia Pacific Women’s Health Market Share Forecast by Distribution Channel, 2023 & 2033 (%)
Figure 105 Japan Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 106 Japan Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 107 Japan Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 108 China Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 109 China Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 110 China Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 111 India Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 112 India Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 113 India Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 114 South Korea Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 115 South Korea Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 116 South Korea Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 117 Australia Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 118 Australia Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 119 Australia Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 120 Rest of Asia Pacific Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 121 Rest of APAC Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 122 Rest of APAC Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 123 Latin America Women’s Health Market Attractiveness Index, 2023
Figure 124 Latin America Women’s Health Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 125 Latin America Women’s Health Market Forecast by Country, 2023-2033 (US$ million, AGR %)
Figure 126 Latin America Women’s Health Market Share Forecast by Country, 2023 & 2033 (%)
Figure 127 Latin America Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 128 Latin America Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 129 Latin America Women’s Health Market Forecast by Distribution Channel, 2023-2033 (US$ million, AGR %)
Figure 130 Latin America Women’s Health Market Share Forecast by Distribution Channel, 2023 & 2033 (%)
Figure 131 Brazil Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 132 Brazil Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 133 Brazil Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 134 Mexico Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 135 Mexico Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 136 Mexico Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 137 Rest of LATAM Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 138 Rest of LATAM Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 139 Rest of LATAM Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 140 MEA Women’s Health Market Attractiveness Index, 2023
Figure 141 MEA Women’s Health Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 142 MEA Women’s Health Market Forecast by Country, 2023-2033 (US$ million, AGR %)
Figure 143 MEA Women’s Health Market Share Forecast by Country, 2023 & 2033 (%)
Figure 144 MEA Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 145 MEA Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 146 MEA Women’s Health Market Forecast by Distribution Channel, 2023-2033 (US$ million, AGR %)
Figure 147 MEA Women’s Health Market Share Forecast by Distribution Channel, 2023 & 2033 (%)
Figure 148 South Africa Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 149 South Africa Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 150 South Africa Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 151 GCC Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 152 GCC Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 153 GCC Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 154 Rest of MEA Women’s Health Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 155 Rest of MEA Women’s Health Market Forecast by Disease, 2023-2033 (US$ million, AGR %)
Figure 156 Rest of MEA Women’s Health Market Share Forecast by Disease, 2023 & 2033 (%)
Figure 157 Global Women’s Health Market: Company Share Analysis, 2021
Figure 158 Pfizer: Net Revenue, 2019-2021 (US$ million, AGR%)
Figure 159 Pfizer: Regional Market Shares, 2021
Figure 160 Pfizer: Gross Profit, 2019-2021 (US$ million, AGR%)
Figure 161 Pfizer: R&D, 2019-2021 (US$ million, AGR%)
Figure 162 Merck: Net Revenue, 2019-2021 (US$ million, AGR%)
Figure 163 Merck: Regional Market Shares, 2021
Figure 164 Merck: Income from Continuing Operations Before Taxes, 2019-2021 (US$ million, AGR%)
Figure 165 Merck: R&D, 2019-2021 (US$ million, AGR%)
Figure 166 Bayer Pharmaceuticals: Net Revenue, 2019-2021 (US$ million, AGR%)
Figure 167 Bayer Pharmaceuticals: Regional Market Shares, 2021
Figure 168 Bayer Pharmaceuticals: EBITDA, 2019-2021 (US$ million, AGR%)
Figure 169 Bayer Pharmaceuticals: R&D, 2019-2021 (US$ million, AGR%)
Figure 170 Eli Lilly and Company: Net Revenue, 2019-2021 (US$ million, AGR%)
Figure 171 Eli Lilly and Company: Regional Market Shares, 2021
Figure 172 Eli Lilly and Company: Net Income, 2019-2021 (US$ million, AGR%)
Figure 173 Eli Lilly and Company: R&D, 2019-2021 (US$ million, AGR%)
Figure 174 Amgen: Net Revenue, 2019-2021 (US$ million, AGR%)
Figure 175 Amgen: Regional Market Shares, 2021
Figure 176 Amgen: Gross Profit, 2019-2021 (US$ million, AGR%)
Figure 177 Amgen: R&D, 2019-2021 (US$ million, AGR%)
Figure 178 AbbVie: Net Revenue, 2019-2021 (US$ million, AGR%)
Figure 179 AbbVie: Regional Market Shares, 2021
Figure 180 AbbVie: Operating Earnings, 2019-2021 (US$ million, AGR%)
Figure 181 AbbVie: R&D, 2019-2021 (US$ million, AGR%)
Figure 182 Novartis: Net Revenue, 2019-2021 (US$ million, AGR%)
Figure 183 Novartis: Regional Market Shares, 2021
Figure 184 Novartis: Gross Profit, 2019-2021 (US$ million, AGR%)
Figure 185 Novartis: R&D, 2019-2021 (US$ million, AGR%)
Figure 186 Agile Therapeutics: Net Revenue, 2019-2021 (US$ million, AGR%)
Figure 187 Agile Therapeutics: Gross Profit, 2019-2021 (US$ million, AGR%)
Figure 188 Agile Therapeutics: R&D, 2019-2021 (US$ million, AGR%)
Figure 189 Ferring B.V.: Net Revenue, 2019-2021 (US$ million, AGR%)
Figure 190 Ferring B.V.: Regional Market Shares, 2021
Figure 191 Ferring B.V.: Gross Profit, 2019-2021 (US$ million, AGR%)
Figure 192 Ferring B.V.: R&D, 2019-2021 (US$ million, AGR%)
ADAllen Pharma
AmerisourceBergen Corporation
Ancillare
Biocair
Catalent, Inc.
Clinigen Group plc
Coghlan Group
COREX Logistics
DHL
Durbin
Endpoint Clinical
Eurofins Scientific SE
Inizio (UDG Healthcare plc)
IQVIA Inc.
KLIFO
Liveo Research
Lonza
Marken (A UPS Company)
Movianto
Myonex
N-SIDE
Parexel
PCI Pharma Services
Piramal Pharma Solutions
Recipharm AB
Rubicon Research Pvt S.A.
Seveillar Clinical Supplies Services
Sharp Corporation
SIRO Clinpharm
Thermo Fisher Scientific Inc.
List of Other Companies Mentioned in the Report
Ahma Group
ALSA Ventures
Annexus Health
Aport
Aragen Life Sciences
Arranta Bio
BeiGene
BeiGene, Ltd.
Berlinger & Co.
Bidco
BioGeneration Ventures
Cedra Express
Clalit
Codiak BioSciences
CTRx Europe
Deestone Group
DisperSol Technologies
DPD Group
Eyevance Pharmaceuticals
Forbion
HealthCore, Inc.
Hubertus
Innoplexus
Intox Pvt. Ltd.
Japan Analytical Chemistry Consultants Co. Ltd.
Kaged Muscle
Kelun-Biotech
Lemonex
Lifecodexx AG
Linehaul Express India Pvt Ltd
Meditab Specialities Ltd.
Megalab SA
Myonex
NeoGenomics, Inc.
NG BV
n-Lorem
NRx Pharmaceuticals
Parsolex
PeproTech
Phanes Therapeutics, Inc.
Phathom Pharmaceuticals
Pinteon
Q BioMed Inc.
Secura Bio, Inc.
Shertech
Stevanato Group S.p.A.
Südpack Medica AG
Svizz-One Corporation Ltd
The Grifols company
Touchlight
Tsing Hua
Viatris
Vibalogics
Villapharma
Waigaoqiao Free Trade Zone (FTZ)
Wellesta Holdings
Yapan Bio
List of Associations Mentioned in the Report
Agencia Espaola de Medicamentos y Productos Sanitarios (AEMPS)
Agência Nacional de Vigilância Sanitária (ANVISA)
Central Drugs Standard Control Organization (CDSCO)
Centro Nacional de Investigaciones Cardiovasculares Carlos III
Centro Studi Assobiotec's
China FDA (CFDA)
Chinese Ministry of Health
Comissão Nacional de Ética em Pesquisa (CONEP)
Consorcio de Apoyo a la Investigación Biomédica – Plataforma Española de Ensayos Clínicos (CAIBER)
Drug Controller General of India (DCGI)
European Commission
European Medicines Agency (EMA)
Food and Drug Administration (FDA)
Health Science Authority
Institute Nehring GmbH
International Air Transport Association (IATA)
International Clinical Multicentre's Trials (IMCT)
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)
International Pharmaceutical Federation
Japanese Ministry of Health, Labour and Welfare (MHLW)
Les Enterprises du Medicament (LEEM)
Medicines and Healthcare Products Regulatory Agency (MHRA)
National Heart, Lung, and Blood Institute
National Institutes of Health (NIH)
Pharmaceuticals for Human Use (ICH)
Transportation Administration Security (TAS)
US Pharmacopeia (USP)
World Health Organization (WHO)
Download sample pages
Complete the form below to download your free sample pages for Clinical Trial Supplies Market Report 2023-2033
Related reports
-

Cell & Gene Therapy Cold Chain Logistics Market Report 2023-2033
The global Cell & Gene Therapy Cold Chain Logistics market is projected to grow at a CAGR of 15% by...Full DetailsPublished: 23 May 2023 -

Paediatric Clinical Trials Market Report 2023-2033
The global Paediatric Clinical Trials market is projected to grow at a CAGR of 6.3% by 2033...Full DetailsPublished: 26 April 2023 -

Top 50 Pharmaceutical Contract Manufacturing Organisations (CMOs) Market Report 2022
Pharmaceutical CMOs market is projected to grow at a CAGR of 6.9% by 2032....Full DetailsPublished: 25 November 2022 -

Decentralized Clinical Trials Market Report 2022-2032
Some of the factors driving the market growth are the rising geriatric population, increasing prevalence of cancer, and rising demand...Full DetailsPublished: 24 January 2022 -

Immuno-Oncology Clinical Trials Market Report 2023-2033
The global Immuno-Oncology Clinical Trials market is projected to grow at a CAGR of 11.3% by 2033...Full DetailsPublished: 11 September 2023 -

Clinical Trial Supply and Logistics Market for Pharma 2023-2033
The global Clinical Trial Supply and Logistics for Pharma market is projected to grow at a CAGR of 8.4% by...Full DetailsPublished: 25 September 2023 -

Fill-Finish Manufacturing Market Report 2023-2033
The global Fill-Finish Manufacturing market is projected to grow at a CAGR of 9.2% by 2033...Full DetailsPublished: 10 February 2023 -

Gene Therapy R&D Market Report 2022-2032
The gene therapy R&D market was valued at US$1,653.0 million in 2021 and is projected to grow at a CAGR...Full DetailsPublished: 15 September 2022 -

Cell and Gene Supply Chain Services and Solutions Market Report 2020-2030
Where is the Cell and Gene Supply Chain Services and Solutions market heading? If you are involved in this sector...Full DetailsPublished: 03 August 2020 -

eClinical Solutions Market Report 2023-2033
The global eClinical Solutions market is projected to grow at a CAGR of 13.09% by 2033...Full DetailsPublished: 17 March 2023
Download sample pages
Complete the form below to download your free sample pages for Clinical Trial Supplies Market Report 2023-2033
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Latest Pharma news
Visiongain Publishes Drug Delivery Technologies Market Report 2024-2034
The global Drug Delivery Technologies market is estimated at US$1,729.6 billion in 2024 and is projected to grow at a CAGR of 5.5% during the forecast period 2024-2034.
23 April 2024
Visiongain Publishes Cell Therapy Technologies Market Report 2024-2034
The cell therapy technologies market is estimated at US$7,041.3 million in 2024 and is projected to grow at a CAGR of 10.7% during the forecast period 2024-2034.
18 April 2024
Visiongain Publishes Automation in Biopharma Industry Market Report 2024-2034
The global Automation in Biopharma Industry market is estimated at US$1,954.3 million in 2024 and is projected to grow at a CAGR of 7% during the forecast period 2024-2034.
17 April 2024
Visiongain Publishes Anti-obesity Drugs Market Report 2024-2034
The global Anti-obesity Drugs market is estimated at US$11,540.2 million in 2024 and is expected to register a CAGR of 21.2% from 2024 to 2034.
12 April 2024


















