The Clinical Trials Support Services Market Report 2023-2033: This report will prove invaluable to leading firms striving for new revenue pockets if they wish to better understand the industry and its underlying dynamics. It will be useful for companies that would like to expand into different industries or to expand their existing operations in a new region.
Factors such as R&D, Clinical Trials Outsourcing and Globalization Driving Industry Growth
The market for clinical trials support services has witnessed substantial growth in recent times, with several pivotal factors shaping the pharmaceutical and biotechnology research landscape. Among the key drivers is the escalating demand for innovative and specialized treatments, which has led to a significant rise in clinical trial activities. As pharmaceutical companies strive to develop cutting-edge therapies to address unmet medical needs, the need for comprehensive clinical trials support services has surged considerably.
Moreover, the increasing prevalence of chronic illnesses and the aging population have necessitated broader and more diverse clinical trials. Conditions like cancer, cardiovascular disorders, and neurological ailments have seen a marked increase, prompting the requirement for larger and more intricate trials to assess the safety and effectiveness of potential treatments. Consequently, pharmaceutical and biotechnology firms are enlisting the assistance of specialized clinical trial support service providers to navigate the complexities of these trials more effectively.
Additionally, the growing focus on adhering to regulatory standards and ensuring data quality has fuelled the demand for specialized expertise and technological advancements in the clinical trial domain. Regulatory authorities worldwide have tightened their scrutiny of clinical trial data, making it crucial for companies to uphold the highest quality standards. As a result, the adoption of advanced technologies and data management solutions, such as electronic data capture (EDC) and clinical trial management systems (CTMS), has become pivotal to guarantee data accuracy, security, and regulatory compliance.
Collectively, these drivers have played a significant role in propelling the notable growth of the clinical trials support services market. As the healthcare landscape continues to evolve and progress, the demand for specialized and comprehensive clinical trial services is projected to further increase, offering fresh prospects and challenges for companies operating within this sector.
Data Quality and Management Issues such as Errors, Omissions, and Validation Gaps Risk Patient Safety and Trial Efficiency
In recent years, the clinical trials support services market has experienced remarkable growth, driven by a rising demand for outsourced solutions to streamline and expedite drug development processes. Nevertheless, the industry faces several challenges, and a significant impediment relates to the management and quality of data. The accuracy and reliability of data play a crucial role in clinical trials, forming the foundation for well-informed decision-making and ensuring precise outcomes and conclusions.
Various factors contribute to data quality issues, including errors during data collection, incomplete or missing data, inadequate validation processes, and inconsistencies in data entry. These factors can compromise the overall integrity of the trial, leading to erroneous conclusions and, more importantly, potentially jeopardizing the safety of patients. Additionally, poorly managed data can lead to delays in project timelines and escalated costs, negatively impacting the efficiency of the entire trial process.
Moreover, data management concerns encompass the need for data security and the assurance that patient information remains confidential and compliant with privacy regulations. Inadequate data protection measures may expose sensitive information to unauthorized access, leading to breaches and potential legal repercussions.
What Questions Should You Ask before Buying a Market Research Report?
• How is the clinical trials support services market evolving?
• What is driving and restraining the clinical trials support services market?
• How will each clinical trials support services market segment grow over the forecast period and how much revenue will these submarkets account for in 2033?
• How will the market shares for each clinical trials support services market submarket develop from 2023 to 2033?
• What will be the main driver for the overall market from 2023 to 2033?
• Will leading clinical trials support services markets broadly follow the macroeconomic dynamics, or will individual national markets outperform others?
• How will the market shares of the national markets change by 2033 and which geographical region will lead the market in 2033?
• Who are the leading players and what are their prospects over the forecast period?
• How will the industry evolve during the period between 2023 and 2033? What are the implications of clinical trials support services projects taking place now and over the next 10 years?
• Is there a greater need for product commercialisation to further scale the clinical trials support services market?
• Where is the clinical trials support services market heading and how can you ensure you are at the forefront of the market?
• What are the best investment options for new product and service lines?
• What are the key prospects for moving companies into a new growth path and C-suite?
You need to discover how this will impact the clinical trials support services market today, and over the next 10 years:
• Our 262-page report provides 99 tables, 132 charts/graphs exclusively to you.
• The report highlights key lucrative areas in the industry so you can target them – NOW.
• It contains in-depth analysis of global, regional and national sales and growth.
• It highlights for you the key successful trends, changes and revenue projections made by your competitors.
This report tells you TODAY how the clinical trials support services market will develop in the next 10 years, and in line with the variations in COVID-19 economic recession and bounce. This market is more critical now than at any point over the last 10 years.
Forecasts to 2033 and other analyses reveal commercial prospects
• In addition to revenue forecasting to 2033, our new study provides you with recent results, growth rates, and market shares.
• You will find original analyses, with business outlooks and developments.
• Discover qualitative analyses (including market dynamics, drivers, opportunities, restraints and challenges), cost structure, impact of rising clinical trials support services prices and recent developments.
This report includes data analysis and invaluable insight into how COVID-19 will affect the industry and your company. Four COVID-19 recovery patterns and their impact, namely, “V”, “L”, “W” and “U” are discussed in this report.
By Service
• Clinical trial site management
• Patient recruitment management
• Data Management and Electronic Data Capture (EDC) Services
• IRB (Institutional Review Boards)
• Others
Phase
• Phase I
• Phase II
• Phase III
• Phase IV
Sponsor
• Pharmaceutical & Biopharmaceutical Companies
• Medical Device Manufacturers
• Others

In addition to the revenue predictions for the overall world market and segments, you will also find revenue forecasts for four regional and 20 leading national markets:
North America
• U.S.
• Canada
Europe
• Germany
• Spain
• UK
• France
• Italy
• Rest of Europe
Asia Pacific
• China
• Japan
• India
• Australia
• South Korea
• Rest of Asia Pacific
Latin America
• Brazil
• Mexico
• Rest of Latin America
MEA
• GCC Countries
• South Africa
• Rest of MEA
Need industry data? Please contact us today.
The report also includes profiles and for some of the leading companies in the Clinical Trials Support Services Market, 2023 to 2033, with a focus on this segment of these companies’ operations.
Leading companies and the potential for market growth
• Caidya
• Calyx
• Eurofins Scientific
• ICON plc
• IQVIA Inc.
• Laboratory Corporation of America
• Merative
• Parexel International Corporation
• Thermo Fisher Scientific Inc.
• WuXi AppTec
Overall world revenue for Clinical Trials Support Services Market, 2023 to 2033 in terms of value the market will surpass US$21 billion in 2023, our work calculates. We predict strong revenue growth through to 2033. Our work identifies which organizations hold the greatest potential. Discover their capabilities, progress, and commercial prospects, helping you stay ahead.
How will the Clinical Trials Support Services Market, 2023 to 2033 report help you?
In summary, our 260+ page report provides you with the following knowledge:
• Revenue forecasts to 2033 for Clinical Trials Support Services Market 2023 to 2033, with forecasts for service, phase, and sponsor each forecast at a global and regional level – discover the industry’s prospects, finding the most lucrative places for investments and revenues.
• Revenue forecasts to 2033 for four regional and 20 key national markets – See forecasts for the Clinical Trials Support Services Market, 2023 to 2033 market in North America, Europe, Asia-Pacific, Latin America and MEA. Also forecasted is the market in the US, Canada, Mexico, Brazil, Germany, France, UK, Italy, China, India, Japan, and Australia among other prominent economies.
• Prospects for established firms and those seeking to enter the market – including company profiles for 10 of the major companies involved in the Clinical Trials Support Services Market, 2023 to 2033.
Find quantitative and qualitative analyses with independent predictions. Receive information that only our report contains, staying informed with invaluable business intelligence.
Information found nowhere else
With our new report, you are less likely to fall behind in knowledge or miss out on opportunities. See how our work could benefit your research, analyses, and decisions. Visiongain’s study is for everybody needing commercial analyses for the Clinical Trials Support Services Market, 2023 to 2033, market-leading companies. You will find data, trends and predictions.
To access the data contained in this document please email contactus@visiongain.com
Buy our report today Clinical Trials Support Services Market Report 2023-2033: Forecasts by Service (Clinical Trial Site Management, Patient Recruitment Management, Data Management and Electronic Data Capture (EDC) Services, IRB (Institutional Review Boards), Others)), by Phase (Phase I, Phase II, Phase III, Phase IV), by Sponsor (Pharmaceutical & Biopharmaceutical Companies, Medical Device Manufacturers, Others) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Impact and Recovery Pattern Analysis. Avoid missing out by staying informed – order our report now.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for a specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: contactus@visiongain.com
1 Report Overview
1.1 Objectives of the Study
1.2 Introduction to Clinical Trials Support Services Market
1.3 What This Report Delivers
1.4 Why You Should Read This Report
1.5 Key Questions Answered by This Analytical Report
1.6 Who is This Report for?
1.7 Methodology
1.7.1 Market Definitions
1.7.2 Market Evaluation & Forecasting Methodology
1.7.3 Data Validation
1.7.3.1 Primary Research
1.7.3.2 Secondary Research
1.8 Frequently Asked Questions (FAQs)
1.9 Associated Visiongain Reports
1.10 About Visiongain
2 Executive Summary
3 Market Overview
3.1 Key Findings
3.2 Market Dynamics
3.2.1 Market Driving Factors
3.2.1.1 Increasing R&D Activities and Increasing Clinical Trials Activities
3.2.1.2 Outsourcing of Clinical Trials Activities Driving Industry Growth
3.2.1.3 Globalization of Clinical Trials Projected to Boost Industry Growth
3.2.2 Market Restraining Factors
3.2.2.1 Strict Regulatory and High Compliance Complexity Likely to Hamper Industry Growth
3.2.2.2 Data Quality and Management Issues Reducing Reliability of Data
3.2.2.3 Patient Recruitment and Retention Over a Diverse Patient Population
3.2.3 Market Opportunities
3.2.3.1 Patient-centric Approaches and Technological Advancements Projected to Offer Lucrative Growth Prospects
3.2.3.2 Precision Medicine and Biomarker-driven Trials
3.2.3.3 Virtual and Decentralized Trials Offering Remote Monitoring, Telemedicine Solutions, and Digital Patient Engagement Platforms
3.3 COVID-19 Impact Analysis
3.4 Porter’s Five Forces Analysis
3.4.1 Bargaining Power of Suppliers
3.4.2 Bargaining Power of Buyers
3.4.3 Competitive Rivalry
3.4.4 Threat from Substitutes
3.4.5 Threat of New Entrants
3.5 PEST Analysis
3.5.1 Political
3.5.2 Economical
3.5.3 Social
3.5.4 Technological
4 Clinical Trials Support Services Market Analysis by Service
4.1 Key Findings
4.2 Service Segment: Market Attractiveness Index
4.3 Clinical Trials Support Services Market Size Estimation and Forecast by Service
4.4 Clinical Trial Site Management
4.4.1 Market Size by Region, 2023-2033 (US$ Billion)
4.4.2 Market Share by Region, 2023 & 2033 (%)
4.5 Patient Recruitment Management
4.5.1 Market Size by Region, 2023-2033 (US$ Billion)
4.5.2 Market Share by Region, 2023 & 2033 (%)
4.5.3 Patient Recruitment Management in Clinical Trials Support Services Market
4.6 Data Management and Electronic Data Capture (EDC) Services
4.6.1 Market Size by Region, 2023-2033 (US$ Billion)
4.6.2 Market Share by Region, 2023 & 2033 (%)
4.7 IRB (Institutional Review Boards)
4.7.1 Market Size by Region, 2023-2033 (US$ Billion)
4.7.2 Market Share by Region, 2023 & 2033 (%)
4.8 Others
4.8.1 Market Size by Region, 2023-2033 (US$ Billion)
4.8.2 Market Share by Region, 2023 & 2033 (%)
5 Clinical Trials Support Services Market Analysis by Phase
5.1 Key Findings
5.2 Phase Segment: Market Attractiveness Index
5.3 Clinical Trials Support Services Market Size Estimation and Forecast by Phase
5.4 Phase I
5.4.1 Market Size by Region, 2023-2033 (US$ Billion)
5.4.2 Market Share by Region, 2023 & 2033 (%)
5.5 Phase II
5.5.1 Market Size by Region, 2023-2033 (US$ Billion)
5.5.2 Market Share by Region, 2023 & 2033 (%)
5.6 Phase III
5.6.1 Market Size by Region, 2023-2033 (US$ Billion)
5.6.2 Market Share by Region, 2023 & 2033 (%)
5.7 Phase IV
5.7.2 Market Size by Region, 2023-2033 (US$ Billion)
5.7.3 Market Share by Region, 2023 & 2033 (%)
6 Clinical Trials Support Services Market Analysis by Sponsor
6.1 Key Findings
6.2 Sponsor Segment: Market Attractiveness Index
6.3 Clinical Trials Support Services Market Size Estimation and Forecast by Sponsor
6.4 Pharmaceutical & Biopharmaceutical Companies
6.4.1 Market Size by Region, 2023-2033 (US$ Billion)
6.4.2 Market Share by Region, 2023 & 2033 (%)
6.5 Medical Device Manufacturers
6.5.2 Market Size by Region, 2023-2033 (US$ Billion)
6.5.3 Market Share by Region, 2023 & 2033 (%)
6.6 Others
6.6.1 Market Size by Region, 2023-2033 (US$ Billion)
6.6.2 Market Share by Region, 2023 & 2033 (%)
7 Clinical Trials Support Services Market Analysis by Region
7.1 Key Findings
7.3 Regional Market Size Estimation and Forecast
8 North America Clinical Trials Support Services Market Analysis
8.1 Key Findings
8.2 North America Clinical Trials Support Services Market Attractiveness Index
8.3 North America Clinical Trials Support Services Market by Country, 2023, 2028 & 2033 (US$ Billion)
8.4 North America Clinical Trials Support Services Market Size Estimation and Forecast by Country
8.5 North America Clinical Trials Support Services Market Size Estimation and Forecast by Service
8.5.1 North America Patient Recruitment Management in Clinical Trials Support Services Market Size Estimation and Forecast by Services
8.6 North America Clinical Trials Support Services Market Size Estimation and Forecast by Phase
8.7 North America Clinical Trials Support Services Market Size Estimation and Forecast by Sponsor
8.8 U.S. Clinical Trials Support Services Market Analysis
8.9 Canada Clinical Trials Support Services Market Analysis
9 Europe Clinical Trials Support Services Market Analysis
9.1 Key Findings
9.2 Europe Clinical Trials Support Services Market Attractiveness Index
9.3 Europe Clinical Trials Support Services Market by Country, 2023, 2028 & 2033 (US$ Billion)
9.4 Europe Clinical Trials Support Services Market Size Estimation and Forecast by Country
9.5 Europe Clinical Trials Support Services Market Size Estimation and Forecast by Service
9.5.1 Europe Patient Recruitment Management in Clinical Trials Support Services Market Size Estimation and Forecast by Services
9.6 Europe Clinical Trials Support Services Market Size Estimation and Forecast by Phase
9.7 Europe Clinical Trials Support Services Market Size Estimation and Forecast by Sponsor
9.8 Germany Clinical Trials Support Services Market Analysis
9.9 France Clinical Trials Support Services Market Analysis
9.10 UK Clinical Trials Support Services Market Analysis
9.11 Italy Clinical Trials Support Services Market Analysis
9.12 Spain Clinical Trials Support Services Market Analysis
9.13 Rest of Europe Clinical Trials Support Services Market Analysis
10 Asia Pacific Clinical Trials Support Services Market Analysis
10.1 Key Findings
10.2 Asia Pacific Clinical Trials Support Services Market Attractiveness Index
10.3 Asia Pacific Clinical Trials Support Services Market by Country, 2023, 2028 & 2033 (US$ Billion)
10.4 Asia Pacific Clinical Trials Support Services Market Size Estimation and Forecast by Country
10.5 Asia Pacific Clinical Trials Support Services Market Size Estimation and Forecast by Service
10.5.1 Asia Pacific Patient Recruitment Management in Clinical Trials Support Services Market Size Estimation and Forecast by Services
10.6 Asia Pacific Clinical Trials Support Services Market Size Estimation and Forecast by Phase
10.7 Asia Pacific Clinical Trials Support Services Market Size Estimation and Forecast by Sponsor
10.8 China Clinical Trials Support Services Market Analysis
10.9 Japan Clinical Trials Support Services Market Analysis
10.10 India Clinical Trials Support Services Market Analysis
10.11 Australia Clinical Trials Support Services Market Analysis
10.12 South Korea Clinical Trials Support Services Market Analysis
10.13 Rest of Asia Pacific Clinical Trials Support Services Market Analysis
11 Latin America Clinical Trials Support Services Market Analysis
11.1 Key Findings
11.2 Latin America Clinical Trials Support Services Market Attractiveness Index
11.3 Latin America Clinical Trials Support Services Market by Country, 2023, 2028 & 2033 (US$ Billion)
11.4 Latin America Clinical Trials Support Services Market Size Estimation and Forecast by Country
11.5 Latin America Clinical Trials Support Services Market Size Estimation and Forecast by Service
11.5.1 Latin America Patient Recruitment Management in Clinical Trials Support Services Market Size Estimation and Forecast by Services
11.6 Latin America Clinical Trials Support Services Market Size Estimation and Forecast by Phase
11.7 Latin America Clinical Trials Support Services Market Size Estimation and Forecast by Sponsor
11.8 Brazil Clinical Trials Support Services Market Analysis
11.9 Mexico Clinical Trials Support Services Market Analysis
11.10 Rest of Latin America Clinical Trials Support Services Market Analysis
12 MEA Clinical Trials Support Services Market Analysis
12.1 Key Findings
12.2 MEA Clinical Trials Support Services Market Attractiveness Index
12.3 MEA Clinical Trials Support Services Market by Country, 2023, 2028 & 2033 (US$ Billion)
12.4 MEA Clinical Trials Support Services Market Size Estimation and Forecast by Country
12.5 MEA Clinical Trials Support Services Market Size Estimation and Forecast by Service
12.5.1 MEA Patient Recruitment Management in Clinical Trials Support Services Market Size Estimation and Forecast by Services
12.6 MEA Clinical Trials Support Services Market Size Estimation and Forecast by Phase
12.7 MEA Clinical Trials Support Services Market Size Estimation and Forecast by Sponsor
12.8 GCC Clinical Trials Support Services Market Analysis
12.9 South Africa Clinical Trials Support Services Market Analysis
12.10 Rest of MEA Clinical Trials Support Services Market Analysis
13 Company Profiles
13.1 Competitive Landscape, 2022
13.2 Strategic Outlook
13.3 Thermo Fisher Scientific Inc.
13.3.1 Company Snapshot
13.3.2 Company Overview
13.3.3 Financial Analysis
13.3.3.1 Net Revenue, 2018-2022
13.3.3.2 Regional Market Shares, 2022
13.3.4 Services Offered
13.3.5 Strategic Outlook
13.4 Eurofins Scientific
13.4.1 Company Snapshot
13.4.2 Company Overview
13.4.3 Financial Analysis
13.4.3.1 Net Revenue, 2018-2022
13.4.3.2 Regional Market Shares, 2022
13.4.4 Services Offered
13.4.5 Strategic Outlook
13.5 IQVIA Inc.
13.5.1 Company Snapshot
13.5.2 Company Overview
13.5.3 Financial Analysis
13.5.3.1 Revenue, 2018-2022
13.5.3.2 Regional Market Shares, 2022
13.5.3.3 R&D Expense, 2018-2022
13.5.4 Services Offered
13.5.5 Strategic Outlook
13.6 WuXi AppTec
13.6.1 Company Snapshot
13.6.2 Company Overview
13.6.3 Financial Analysis
13.6.3.1 Revenue, 2019-2022
13.6.3.2 Regional Market Shares, 2022
13.6.4 Services Offered
13.6.5 Strategic Outlook
13.7 Parexel International Corporation
13.7.1 Company Snapshot
13.7.2 Company Overview
13.7.3 Services Offered
13.7.4 Strategic Outlook
13.8 Merative
13.8.1 Company Snapshot
13.8.2 Company Overview
13.8.3 Services Offered
13.8.4 Strategic Outlook
13.9 Calyx
13.9.1 Company Snapshot
13.9.2 Company Overview
13.9.3 Services Offered
13.9.4 Strategic Outlook
13.10 Caidya
13.10.1 Company Snapshot
13.10.2 Company Overview
13.10.3 Services Offered
13.10.4 Strategic Outlook
13.11 Laboratory Corporation of America
13.11.1 Company Snapshot
13.11.2 Company Overview
13.11.3 Financial Analysis
13.11.3.1 Revenue, 2018-2022
13.11.3.2 Regional Market Shares, 2022
13.11.4 Services Offered
13.11.5 Strategic Outlook
13.12 ICON plc
13.12.1 Company Snapshot
13.12.2 Company Overview
13.12.3 Financial Analysis
13.12.3.1 Revenue, 2018-2022
13.12.3.2 Regional Market Shares, 2022
13.12.4 Services Offered
13.12.5 Strategic Outlook
14 Conclusion and Recommendations
14.1 Concluding Remarks from Visiongain
14.2 Recommendations for Market Players
List of Tables
Table 1 Clinical Trials Support Services Market Snapshot, 2023 & 2033 (US$ billion, CAGR %)
Table 2 Total Clinical Trials by Phase of Development
Table 3 Clinical Trials Support Services Market Forecast by Region 2023-2033 (US$ Bn, AGR%, CAGR%): "V" Shaped Recovery
Table 4 Clinical Trials Support Services Market Forecast by Region 2023-2033 (US$ Bn, AGR%, CAGR%): "U" Shaped Recovery
Table 5 Clinical Trials Support Services Market Forecast by Region 2023-2033 (US$ Bn, AGR%, CAGR%): "W" Shaped Recovery
Table 6 Clinical Trials Support Services Market Forecast by Region 2023-2033 (US$ Bn, AGR%, CAGR%): "L" Shaped Recovery
Table 7 Global Clinical Trials Support Services Market Forecast by Services, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 8 Clinical Trial Site Management Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 9 Patient Recruitment Management Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 10 Patient Recruitment Management in Clinical Trials Support Services Market Forecast by Services, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 11 Data Management and Electronic Data Capture (EDC) Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 12 IRB (Institutional Review Boards) Segment Market Forecast by Region, 2023-2033
(US$ Billion, AGR%, CAGR%)
Table 13 Others Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 14 Global Clinical Trials Support Services Market Forecast by Phase, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 15 Phase I Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 16 Phase II Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 17 Phase III Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 18 Phase IV Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 19 Global Clinical Trials Support Services Market Forecast by Sponsor, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 20 Pharmaceutical & Biopharmaceutical Companies Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 21 Medical Device Manufacturers Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 22 Others Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 23 Global Clinical Trials Support Services Market Forecast by Region 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 24 North America Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 25 North America Clinical Trials Support Services Market Forecast by Services, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 26 North America Patient Recruitment Management in Clinical Trials Support Services Market Forecast by Services, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 27 North America Clinical Trials Support Services Market Forecast by Phase, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 28 North America Clinical Trials Support Services Market Forecast by Sponsor, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 29 U.S. Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 30 Canada Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 31 Europe Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 32 Europe Clinical Trials Support Services Market Forecast by Services, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 33 Europe Patient Recruitment Management in Clinical Trials Support Services Market Forecast by Services, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 34 Europe Clinical Trials Support Services Market Forecast by Phase, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 35 Europe Clinical Trials Support Services Market Forecast by Sponsor, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 36 Germany Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 37 France Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 38 UK Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 39 Italy Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 40 Spain Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 41 Rest of Europe Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 42 Asia Pacific Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 43 Asia Pacific Clinical Trials Support Services Market Forecast by Services, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 44 Asia Pacific Patient Recruitment Management in Clinical Trials Support Services Market Forecast by Services, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 45 Asia Pacific Clinical Trials Support Services Market Forecast by Phase, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 46 Asia Pacific Clinical Trials Support Services Market Forecast by Sponsor, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 47 China Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 48 Japan Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 49 India Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 50 Australia Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 51 South Korea Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 52 Asia-Pacific Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 53 Latin America Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 54 Latin America Clinical Trials Support Services Market Forecast by Services, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 55 Latin America Patient Recruitment Management in Clinical Trials Support Services Market Forecast by Services, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 56 Latin America Clinical Trials Support Services Market Forecast by Phase, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 57 Latin America Clinical Trials Support Services Market Forecast by Sponsor, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 58 Brazil Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 59 Mexico Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 60 Rest of Latin America Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 61 MEA Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 62 MEA Clinical Trials Support Services Market Forecast by Services, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 63 MEA Patient Recruitment Management in Clinical Trials Support Services Market Forecast by Services, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 64 MEA Clinical Trials Support Services Market Forecast by Phase, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 65 MEA Clinical Trials Support Services Market Forecast by Sponsor, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 66 GCC Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 67 South Africa Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 68 Rest of MEA Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 69 Strategic Outlook
Table 70 Thermo Fisher Scientific Inc: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 71 Thermo Fisher Scientific Inc: Services Offered
Table 72 Thermo Fisher Scientific Inc: Strategic Outlook
Table 73 Eurofins Scientific: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 74 Eurofins Scientific: Services Offered
Table 75 Eurofins Scientific: Strategic Outlook
Table 76 IQVIA Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 77 IQVIA Inc: Services Offered
Table 78 IQVIA Inc: Strategic Outlook
Table 79 WuXi AppTec: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 80 WuXi AppTec: Services Offered
Table 81 WuXi AppTec: Strategic Outlook
Table 82 Parexel International Corporation: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 83 Parexel International Corporation.: Services Offered
Table 84 Parexel International Corporation: Strategic Outlook
Table 85 Merative: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 86 Amcor plc: Services Offered
Table 87 Merative: Strategic Outlook
Table 88 Calyx: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 89 Calyx: Service Benchmarking
Table 90 Calyx: Strategic Outlook
Table 91 Caidya: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 92 Caidya: Service Benchmarking
Table 93 Caidya: Strategic Outlook
Table 94 Laboratory Corporation of America: Key Details, (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 95 Laboratory Corporation of America: Service Benchmarking
Table 96 Laboratory Corporation of America: Strategic Outlook
Table 97 ICON plc: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 98 ICON plc: Services Offered
Table 99 ICON plc: Strategic Outlook
List of Figures
Figure 1 Clinical Trials Support Services Market Segmentation
Figure 2 Clinical Trial Support Market by Service: Market Attractiveness Index
Figure 3 Clinical Trials Support Services Market Forecast by Phase: Market Attractiveness Index
Figure 4 Clinical Trials Support Services Market Forecast by Sponsor: Market Attractiveness Index
Figure 5 Clinical Trials Support Services Market Attractiveness Index by Region
Figure 6 Clinical Trials Support Services Market: Market Dynamics
Figure 7 Clinical Trials Support Services Market by Region, 2023-2033 (US$ Billion, AGR (%), CAGR (%)): “V” Shaped Recovery
Figure 8 Clinical Trials Support Services Market by Region, 2023-2033 (US$ Billion, AGR (%), CAGR (%)): “U” Shaped Recovery
Figure 9 Clinical Trials Support Services Market by Region, 2023-2033 (US$ Billion, AGR (%), CAGR (%)): “W” Shaped Recovery
Figure 10 Clinical Trials Support Services Market by Region, 2023-2033 (US$ Billion, AGR (%), CAGR (%)): “L” Shaped Recovery
Figure 11 Clinical Trials Support Services Market: Porter’s Five Forces Analysis
Figure 12 Clinical Trials Support Services Market: PEST Analysis
Figure 13 Clinical Trials Support Services Market Forecast by Service: Market Attractiveness Index
Figure 14 Clinical Trials Support Services Market Forecast by Service, 2023-2033 (US$ Billion)
Figure 15 Clinical Trials Support Services Market Share Forecast by Service, 2023, 2028, 2033 (%)
Figure 16 Clinical Trial Site Management Market Forecast by Region, 2023 2033 (US$ Billion)
Figure 17 Clinical Trial Site Management Market Share Forecast by Region, 2023 & 2033 (%)
Figure 18 Patient Recruitment Management Market Forecast by Region, 2023-2033 (US$ Billion)
Figure 19 Patient Recruitment Management Market Share Forecast by Region, 2023 & 2033 (%)
Figure 20 Data Management and Electronic Data Capture (EDC) Market Forecast by Region, 2023-2033 (US$ Billion)
Figure 21 Data Management and Electronic Data Capture (EDC) Market Share Forecast by Region, 023 & 2033 (%)
Figure 22 IRB (Institutional Review Boards) Market Forecast by Region, 2023-2033 (US$ Billion)
Figure 23 IRB (Institutional Review Boards) Market Share Forecast by Region, 2023 & 2033 (%)
Figure 24 Others Market Forecast by Region, 2023-2033 (US$ Billion)
Figure 25 Others Market Share Forecast by Region, 2023 & 2033 (%)
Figure 26 Clinical Trials Support Services Market Forecast by Phase: Market Attractiveness Index
Figure 27 Clinical Trials Support Services Market Forecast By Phase, 2023-2033 (US$ Billion)
Figure 28 Clinical Trials Support Services Market Share Forecast by Phase, 2023, 2028, 2033 (%)
Figure 29 Phase I Market Forecast by Region, 2023-2033 (US$ Billion)
Figure 30 Phase I Market Share Forecast by Region, 2023 & 2033 (%)
Figure 31 Phase II Market Forecast by Region, 2023-2033 (US$ Billion)
Figure 32 Phase II Market Share Forecast by Region, 2023 & 2033 (%)
Figure 33 Phase III Market Forecast by Region, 2023-2033 (US$ Billion)
Figure 34 Phase III Market Share Forecast by Region, 2023 & 2033 (%)
Figure 35 Phase IV Market Forecast by Region, 2023-2033 (US$ Billion)
Figure 36 Phase IV Market Share Forecast by Region, 2023 & 2033 (%)
Figure 37 Clinical Trials Support Services Market Forecast by Sponsor: Market Attractiveness Index
Figure 38 Clinical Trials Support Services Market Forecast by Sponsor, 2023-2033 (US$ Billion)
Figure 39 Clinical Trials Support Services Market Share Forecast by Sponsor, 2023, 2028, 2033 (%)
Figure 40 Pharmaceutical & Biopharmaceutical Companies Market Forecast by Region, 2023-2033 (US$ Billion)
Figure 41 Pharmaceutical & Biopharmaceutical Companies Market Share Forecast by Region, 2023 & 2033 (%)
Figure 42 Medical Device Manufacturers Market Forecast by Region, 2023-2033 (US$ Billion)
Figure 43 Medical Device Manufacturers Market Share Forecast by Region, 2023 & 2033 (%)
Figure 44 Others Market Forecast by Region, 2023-2033 (US$ Billion)
Figure 45 Others Market Share Forecast by Region, 2023 & 2033 (%)
Figure 46 Clinical Trials Support Services Market Forecast by Region 2023 & 2033 (Revenue, CAGR%)
Figure 47 Clinical Trials Support Services Market Share Forecast by Region 2023, 2028, 2033(%)
Figure 48 Clinical Trials Support Services Market by Region, 2023-2033 (US$ Billion, AGR (%), CAGR (%))
Figure 49 North America Clinical Trials Support Services Market Attractiveness Index
Figure 50 North America Clinical Trials Support Services Market by Region, 2023, 2028 & 2033 (US$ Billion)
Figure 51 North America Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion)
Figure 52 North America Clinical Trials Support Services Market Share Forecast by Country, 2023 & 2033 (%)
Figure 53 North America Clinical Trials Support Services Market Forecast by Service, 2023-2033 (US$ Billion)
Figure 54 North America Clinical Trials Support Services Market Share Forecast by Service, 2023 & 2033 (%)
Figure 55 North America Clinical Trials Support Services Market Forecast by Phase, 2023-2033 (US$ Billion
Figure 56 North America Clinical Trials Support Services Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 57 North America Clinical Trials Support Services Market Forecast by Sponsor, 2023-2033 (US$ Billion)
Figure 58 North America Clinical Trials Support Services Market Share Forecast by Sponsor, 2023 & 2033 (%)
Figure 59 U.S. Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 60 Canada Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 61 Europe Clinical Trials Support Services Market Attractiveness Index
Figure 62 Europe Clinical Trials Support Services Market by Region, 2023, 2028 & 2033 (US$ Billion)
Figure 63 Europe Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion)
Figure 64 Europe Clinical Trials Support Services Market Share Forecast by Country, 2023 & 2033 (%)
Figure 65 Europe Clinical Trials Support Services Market Forecast by Service, 2023-2033 (US$ Billion
Figure 66 Europe Clinical Trials Support Services Market Share Forecast by Service, 2023 & 2033 (%)
Figure 67 Europe Clinical Trials Support Services Market Forecast by Phase, 2023-2033 (US$ Billion)
Figure 68 Europe Clinical Trials Support Services Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 69 Europe Clinical Trials Support Services Market Forecast by Sponsor, 2023-2033 (US$ Billion)
Figure 70 Europe Clinical Trials Support Services Market Share Forecast by Sponsor, 2023 & 2033 (%)
Figure 71 Germany Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 72 France Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 73 UK Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 74 Italy Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 75 Spain Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 76 Rest of Europe Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 77 Asia Pacific Clinical Trials Support Services Market Attractiveness Index
Figure 78 Asia Pacific Clinical Trials Support Services Market by Region, 2023, 2028 & 2033 (US$ Billion)
Figure 79 Asia Pacific Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion)
Figure 80 Asia Pacific Clinical Trials Support Services Market Share Forecast by Country, 2023 & 2033 (%)
Figure 81 Asia Pacific Clinical Trials Support Services Market Forecast by Service, 2023-2033 (US$ Billion
Figure 82 Asia Pacific Clinical Trials Support Services Market Share Forecast by Service, 2023 & 2033 (%)
Figure 83 Asia Pacific Clinical Trials Support Services Market Forecast by Phase, 2023-2033 (US$ Billion)
Figure 84 Asia Pacific Clinical Trials Support Services Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 85 Asia Pacific Clinical Trials Support Services Market Forecast by Sponsor, 2023-2033 (US$ Billion)
Figure 86 Asia Pacific Clinical Trials Support Services Market Share Forecast by Sponsor, 2023 & 2033 (%)
Figure 87 China Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 88 Japan Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 89 India Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 90 Australia Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 91 South Korea Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 92 Rest of Asia Pacific Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 93 Latin America Clinical Trials Support Services Market Attractiveness Index
Figure 94 Latin America Clinical Trials Support Services Market by Region, 2023, 2028 & 2033 (US$ Billion)
Figure 95 Latin America Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion)
Figure 96 Latin America Clinical Trials Support Services Market Share Forecast by Country, 2023 & 2033 (%)
Figure 97 Latin America Clinical Trials Support Services Market Forecast by Service, 2023-2033 (US$ Billion
Figure 98 Latin America Clinical Trials Support Services Market Share Forecast by Service, 2023 & 2033 (%)
Figure 99 Latin America Clinical Trials Support Services Market Forecast by Phase, 2023-2033 (US$ Billion)
Figure 100 Latin America Clinical Trials Support Services Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 101 Latin America Clinical Trials Support Services Market Forecast by Sponsor, 2023-2033 (US$ Billion)
Figure 102 Latin America Clinical Trials Support Services Market Share Forecast by Sponsor, 2023 & 2033 (%)
Figure 103 Brazil Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 104 Mexico Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 105 Rest of Latin America Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 106 MEA Clinical Trials Support Services Market Attractiveness Index
Figure 107 MEA Clinical Trials Support Services Market by Region, 2023, 2028 & 2033 (US$ Billion)
Figure 108 MEA Clinical Trials Support Services Market Forecast by Country, 2023-2033 (US$ Billion)
Figure 109 MEA Clinical Trials Support Services Market Share Forecast by Country, 2023 & 2033 (%)
Figure 110 MEA Clinical Trials Support Services Market Forecast by Service, 2023-2033 (US$ Billion)
Figure 111 MEA Clinical Trials Support Services Market Share Forecast by Service, 2023 & 2033 (%)
Figure 112 MEA Clinical Trials Support Services Market Forecast by Phase, 2023-2033 (US$ Billion)
Figure 113 MEA Clinical Trials Support Services Market Share Forecast by Phase, 2023 & 2033 (%)
Figure 114 MEA Clinical Trials Support Services Market Forecast by Sponsor, 2023-2033 (US$ Billion)
Figure 115 MEA Clinical Trials Support Services Market Share Forecast by Sponsor, 2023 & 2033 (%)
Figure 116 GCC Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 117 South Africa Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
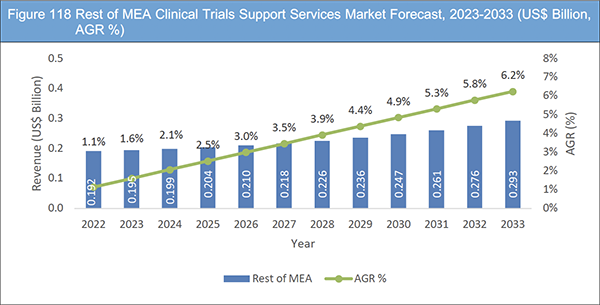
Figure 118 Rest of MEA Clinical Trials Support Services Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 119 Clinical Trials Support Services Market: Company Share Analysis, 2022
Figure 120 Thermo Fisher Scientific Inc: Net Revenue, 2018-2022 (US$ million, AGR%)
Figure 121 Thermo Fisher Scientific Inc: Regional Market Shares, 2022
Figure 122 Eurofins Scientific: Net Revenue, 2018-2022 (US$ million, AGR%)
Figure 123 Eurofins Scientific: Regional Market Shares, 2022
Figure 124 IQVIA Inc: Annual Revenue, 2018-2022 (US$ million, AGR%)
Figure 125 IQVIA Inc: Regional Market Shares, 2022
Figure 126 IQVIA Inc: R&D Expense, 2018-2022 (US$ million, AGR%)
Figure 127 WuXi AppTec: Annual Revenue, 2019-2022 (US$ million, AGR%)
Figure 128 WuXi AppTec: Regional Market Shares, 2022
Figure 129 Laboratory Corporation of America: Revenue, 2018-2022 (US$ Million, AGR%)
Figure 130 Laboratory Corporation of America: Regional Market Shares, 2022
Figure 131 ICON plc: Revenue, 2018-2022 (US$ Billion, AGR%)
Figure 132 ICON plc: Regional Market Shares, 2022
List of Companies Profiled in the report
Caidya
Calyx
Eurofins Scientific
ICON plc
IQVIA Inc.
Laboratory Corporation of America
Merative
Parexel International Corporation
Thermo Fisher Scientific Inc.
WuXi AppTec
List of Other Companies Mentioned in the report
Bioclinica
Catalent, Inc.
CliniSys Group (formerly Eclair Group)
inVentiv Health (now part of Syneos Health)
MedNet Solutions (now part of Bioclinica)
Medpace Holdings, Inc.
PPD, Inc.
PRA Health Sciences
Premier Research
Syneos Health
List of Associations Mentioned in the Report
American Association of Pharmaceutical Scientists (AAPS)
Association of Clinical Research Organizations (ACRO)
Association of Clinical Research Organizations in Austria (ACROA)
Association of Clinical Research Professionals (ACRP)
Association of the British Pharmaceutical Industry (ABPI)
CenterWatch
China Pharmaceutical Innovation and Research Development Association (PhIRDA)
Clinical Data Interchange Standards Consortium (CDISC)
Clinical Research Forum (CR Forum)
Clinical Trials Ontario (CTO)
Clinical Trials Transformation Initiative (CTTI)
Drug Information Association (DIA)
European Clinical Research Infrastructures Network (ECRIN)
Indian Society for Clinical Research (ISCR)
International Society for Pharmacoeconomics and Outcomes Research (ISPOR)
Japan Pharmaceutical Manufacturers Association (JPMA)
National Institute for Health Research (NIHR)
Pharmaceutical Research and Manufacturers of America (PhRMA)
Society for Clinical Data Management (SCDM)
Society for Clinical Trials (SCT)
TransCelerate BioPharma Inc.





























