The Orphan Drugs Market Report 2023-2033: This report will prove invaluable to leading firms striving for new revenue pockets if they wish to better understand the industry and its underlying dynamics. It will be useful for companies that would like to expand into different industries or to expand their existing operations in a new region.
High Profitability Due to Premium Pricing and Regulatory Incentives Projected to Spur Industry Growth
The orphan drugs market is being propelled by a combination of factors. First and foremost, the potential for high profitability stands out as a key driver. Due to the limited patient populations and the specialized nature of rare diseases, pharmaceutical companies can price orphan drugs at premium levels, creating a significant revenue potential. Regulatory incentives, such as extended market exclusivity, reduced regulatory fees, and tax credits, further motivate companies to invest in orphan drug development, making these treatments financially viable.
Additionally, increasing research and development efforts aimed at addressing unmet medical needs in the rare disease space are bolstering market growth. Collaborations between academic institutions, market players, and regulatory authorities are fostering knowledge sharing and accelerating orphan drug development, thereby reducing duplication of efforts and expediting treatments’ translation from research findings.
Furthermore, the rising prevalence of rare diseases in emerging economies, coupled with efforts to improve healthcare infrastructure and awareness, has opened up significant growth opportunities. Governments in developing countries are also implementing favourable policies, such as India’s National Policy for Rare Diseases, which is creating a conducive environment for orphan drug manufacturers and market stakeholders.
Lastly, streamlined regulatory approval processes, which include expedited review pathways and incentives for orphan drug manufacturers, enhance affordability and patient access to these critical treatments, further fueling the orphan drugs market’s expansion.
High Cost Associated with Drug Development Likely to Challenge Industry Growth
The high cost associated with orphan drug development poses a significant challenge to the growth of this market. Developing drugs for rare diseases is often an expensive endeavour due to the limited patient populations available for clinical trials, specialized research requirements, and the need for extensive testing to establish safety and efficacy. These elevated development costs can deter pharmaceutical companies from investing in orphan drug research and development.
Additionally, the smaller patient pool for rare diseases means that the potential revenue from orphan drugs is limited, which makes it challenging to recoup the substantial development expenses. This financial hurdle can discourage companies from pursuing orphan drug projects, particularly when they could allocate resources to more financially rewarding mainstream drug development.
To address this issue, regulatory authorities have introduced incentives such as extended market exclusivity and reduced regulatory fees to make orphan drug development more financially attractive. Nonetheless, the high upfront costs remain a substantial barrier that may hamper the growth of the orphan drugs market and hinder the development of much-needed therapies for rare diseases.
What Questions Should You Ask before Buying a Market Research Report?
• How is the Orphan Drugs market evolving?
• What is driving and restraining the Orphan Drugs market?
• How will each Orphan Drugs submarket segment grow over the forecast period and how much revenue will these submarkets account for in 2033?
• How will the market shares for each Orphan Drugs submarket develop from 2023 to 2033?
• What will be the main driver for the overall market from 2023 to 2033?
• Will leading Orphan Drugs markets broadly follow the macroeconomic dynamics, or will individual national markets outperform others?
• How will the market shares of the national markets change by 2033 and which geographical region will lead the market in 2033?
• Who are the leading players and what are their prospects over the forecast period?
• What are the Orphan Drugs projects for these leading companies?
• How will the industry evolve during the period between 2023 and 2033? What are the implications of Orphan Drugs projects taking place now and over the next 10 years?
• Is there a greater need for product commercialisation to further scale the Orphan Drugs market?
• Where is the Orphan Drugs market heading and how can you ensure you are at the forefront of the market?
• What are the best investment options for new product and service lines?
• What are the key prospects for moving companies into a new growth path and C-suite?
You need to discover how this will impact the Orphan Drugs market today, and over the next 10 years:
• Our 262-page report provides 96 tables and 143 charts/graphs exclusively to you.
• The report highlights key lucrative areas in the industry so you can target them – NOW.
• It contains in-depth analysis of global, regional and national sales and growth.
• It highlights for you the key successful trends, changes and revenue projections made by your competitors.
This report tells you TODAY how the Orphan Drugs market will develop in the next 10 years, and in line with the variations in COVID-19 economic recession and bounce. This market is more critical now than at any point over the last 10 years.
Forecasts to 2033 and other analyses reveal commercial prospects
• In addition to revenue forecasting to 2033, our new study provides you with recent results, growth rates, and market shares.
• You will find original analyses, with business outlooks and developments.
• Discover qualitative analyses (including market dynamics, drivers, opportunities, restraints and challenges), cost structure, impact of rising Orphan Drugs prices and recent developments.
This report includes data analysis and invaluable insight into how COVID-19 will affect the industry and your company. Four COVID-19 recovery patterns and their impact, namely, “V”, “L”, “W” and “U” are discussed in this report.
Segments Covered in the Report
Therapeutic Area
• Cancer
• Neurological Diseases
• Cardiovascular Diseases
• Hematologic Disorders
• Infectious Diseases
• Metabolic Diseases
• Others
Drug Type
• Biologics
• Biosimilar
• Others

In addition to the revenue predictions for the overall world market and segments, you will also find revenue forecasts for five regional and 24 leading national markets:
North America
• U.S.
• Canada
Europe
• Germany
• UK
• France
• Spain
• Italy
• Russia
• Rest of Europe
Asia Pacific
• China
• Japan
• India
• Australia
• South Korea
• Singapore
• Rest of Asia Pacific
Latin America
• Brazil
• Mexico
• Argentina
• Colombia
• Rest of Latin America
MEA
• GCC
• South Africa
• Rest of MEA
Need industry data? Please contact us today.
The report also includes profiles and for some of the leading companies in the Orphan Drugs Market, 2023 to 2033, with a focus on this segment of these companies’ operations.
Leading companies and the potential for market growth
• AbbVie Inc.
• AstraZeneca
• Biogen
• Bristol-Myers Squibb Company
• F. Hoffmann-La Roche Ltd.
• GSK plc
• Johnson & Johnson Services, Inc.
• Merck & Co., Inc.
• Novartis AG
• Pfizer Inc.
• Sanofi
• Vertex Pharmaceuticals Incorporated
Overall world revenue for Orphan Drugs Market, 2023 to 2033 in terms of value the market will surpass US$187 billion in 2023, our work calculates. We predict strong revenue growth through to 2033. Our work identifies which organizations hold the greatest potential. Discover their capabilities, progress, and commercial prospects, helping you stay ahead.
How will the Orphan Drugs Market, 2023 to 2033 report help you?
In summary, our 250+ page report provides you with the following knowledge:
• Revenue forecasts to 2033 for Orphan Drugs Market, 2023 to 2033 Market, with forecasts for therapeutic area, drug type, and brands, each forecast at a global and regional level – discover the industry’s prospects, finding the most lucrative places for investments and revenues.
• Revenue forecasts to 2033 for five regional and 24 key national markets – See forecasts for the Orphan Drugs Market, 2023 to 2033 market in North America, Europe, Asia-Pacific, Latin America, and MEA. Also forecasted is the market in the US, Canada, Mexico, Brazil, Germany, France, UK, Italy, China, India, Japan, and Australia among other prominent economies.
• Prospects for established firms and those seeking to enter the market – including company profiles for 12 of the major companies involved in the Orphan Drugs Market, 2023 to 2033.
Find quantitative and qualitative analyses with independent predictions. Receive information that only our report contains, staying informed with invaluable business intelligence.
Information found nowhere else
With our new report, you are less likely to fall behind in knowledge or miss out on opportunities. See how our work could benefit your research, analyses, and decisions. Visiongain’s study is for everybody needing commercial analyses for the Orphan Drugs Market, 2023 to 2033, market-leading companies. You will find data, trends and predictions.
To access the data contained in this document please email contactus@visiongain.com
Buy our report today Orphan Drugs Market Report 2023-2033: Forecasts by Therapeutic Area (Cancer, Neurological Diseases, Cardiovascular Diseases, Hematologic Disorders, Infectious Diseases, Metabolic Diseases, Others), by Drug Type (Biologics, Biosimilar, Others) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Impact and Recovery Pattern Analysis. Avoid missing out by staying informed – order our report now.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for a specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: contactus@visiongain.com
1 Report Overview
1.1 Objectives of the Study
1.2 Introduction to Orphan Drugs Market
1.3 What This Report Delivers
1.4 Why You Should Read This Report
1.5 Key Questions Answered by This Analytical Report
1.6 Who is This Report for?
1.7 Methodology
1.7.1 Market Definitions
1.7.2 Market Evaluation & Forecasting Methodology
1.7.3 Data Validation
1.7.3.1 Primary Research
1.7.3.2 Secondary Research
1.8 Frequently Asked Questions (FAQs)
1.9 Associated Visiongain Reports
1.10 About Visiongain
2 Executive Summary
3 Market Overview
3.1 Key Findings
3.2 Market Dynamics
3.2.1 Market Driving Factors
3.2.1.1 Market Exclusivity for Orphan Drugs
3.2.1.2 Favourable Government Policies
3.2.1.3 Increasing R&D Activities in the Development of Novel Therapies
3.2.2 Market Restraining Factors
3.2.2.1 High Cost Associated with Drug Development
3.2.2.2 Expiration of Exclusive Marketing Rights for Vital Products
3.2.3 Market Opportunities
3.2.3.1 High Growth Potential in Emerging Economies
3.2.3.2 Use of Orphan Drugs for Novel Indications
3.3 COVID-19 Impact Analysis
3.4 Porter’s Five Forces Analysis
3.4.1 Bargaining Power of Suppliers
3.4.2 Bargaining Power of Buyers
3.4.3 Competitive Rivalry
3.4.4 Threat from Substitutes
3.4.5 Threat of New Entrants
3.5 PEST Analysis
4 Orphan Drugs Market Analysis by Therapeutic Area
4.1 Key Findings
4.2 Therapeutic Area Segment: Market Attractiveness Index
4.3 Orphan Drugs Market Size Estimation and Forecast by Therapeutic Area
4.4 Cancer
4.4.1 Market Size by Region, 2023-2033 (US$ Billion)
4.4.2 Market Share by Region, 2023 & 2033 (%)
4.5 Neurological Diseases
4.5.1 Market Size by Region, 2023-2033 (US$ Billion)
4.5.2 Market Share by Region, 2023 & 2033 (%)
4.6 Cardiovascular Diseases
4.6.1 Market Size by Region, 2023-2033 (US$ Billion)
4.6.2 Market Share by Region, 2023 & 2033 (%)
4.7 Hematologic Disorders
4.7.1 Market Size by Region, 2023-2033 (US$ Billion)
4.7.2 Market Share by Region, 2023 & 2033 (%)
4.8 Infectious Diseases
4.8.1 Market Size by Region, 2023-2033 (US$ Billion)
4.8.2 Market Share by Region, 2023 & 2033 (%)
4.9 Metabolic Diseases
4.9.1 Market Size by Region, 2023-2033 (US$ Billion)
4.9.2 Market Share by Region, 2023 & 2033 (%)
4.10 Others
4.10.1 Market Size by Region, 2023-2033 (US$ Billion)
4.10.2 Market Share by Region, 2023 & 2033 (%)
5 Orphan Drugs Market Analysis by Drug Type
5.1 Key Findings
5.2 Drug Type Segment: Market Attractiveness Index
5.3 Orphan Drugs Market Size Estimation and Forecast by Drug Type
5.4 Biologics
5.4.1 Market Size by Region, 2023-2033 (US$ Billion)
5.4.2 Market Share by Region, 2023 & 2033 (%)
5.5 Biosimilar
5.5.1 Market Size by Region, 2023-2033 (US$ Billion)
5.5.2 Market Share by Region, 2023 & 2033 (%)
5.6 Others
5.6.1 Market Size by Region, 2023-2033 (US$ Billion)
5.6.2 Market Share by Region, 2023 & 2033 (%)
6 Orphan Drugs Market Analysis by Top 10 Brands
6.1 Key Findings
6.2 Orphan Drugs Market Size Estimation and Forecast by Top 10 Brands
7 Orphan Drugs Market Analysis by Region
7.1 Key Findings
7.2 Regional Market Size Estimation and Forecast
8 North America Orphan Drugs Market Analysis
8.1 Key Findings
8.2 North America Orphan Drugs Market Attractiveness Index
8.3 North America Orphan Drugs Market by Country, 2023, 2028 & 2033 (US$ Billion)
8.4 North America Orphan Drugs Market Size Estimation and Forecast by Country
8.5 North America Orphan Drugs Market Size Estimation and Forecast by Therapeutic Area
8.6 North America Orphan Drugs Market Size Estimation and Forecast by Drug Type
8.7 U.S. Orphan Drugs Market Analysis
8.8 Canada Orphan Drugs Market Analysis
9 Europe Orphan Drugs Market Analysis
9.1 Key Findings
9.2 Europe Orphan Drugs Market Attractiveness Index
9.3 Europe Orphan Drugs Market by Country, 2023, 2028 & 2033 (US$ Billion)
9.4 Europe Orphan Drugs Market Size Estimation and Forecast by Country
9.5 Europe Orphan Drugs Market Size Estimation and Forecast by Therapeutic Area
9.6 Europe Orphan Drugs Market Size Estimation and Forecast by Drug Type
9.7 Germany Orphan Drugs Market Analysis
9.8 France Orphan Drugs Market Analysis
9.9 UK Orphan Drugs Market Analysis
9.10 Italy Orphan Drugs Market Analysis
9.11 Spain Orphan Drugs Market Analysis
9.12 Russia Orphan Drugs Market Analysis
9.13 Rest of Europe Orphan Drugs Market Analysis
10 Asia Pacific Orphan Drugs Market Analysis
10.1 Key Findings
10.2 Asia Pacific Orphan Drugs Market Attractiveness Index
10.3 Asia Pacific Orphan Drugs Market by Country, 2023, 2028 & 2033 (US$ Billion)
10.4 Asia Pacific Orphan Drugs Market Size Estimation and Forecast by Country
10.5 Asia Pacific Orphan Drugs Market Size Estimation and Forecast by Therapeutic Area
10.6 Asia Pacific Orphan Drugs Market Size Estimation and Forecast by Drug Type
10.7 Japan Orphan Drugs Market Analysis
10.8 China Orphan Drugs Market Analysis
10.9 India Orphan Drugs Market Analysis
10.10 Australia Orphan Drugs Market Analysis
10.11 South Korea Orphan Drugs Market Analysis
10.12 Singapore Orphan Drugs Market Analysis
10.13 Rest of Asia Pacific Orphan Drugs Market Analysis
11 Latin America Orphan Drugs Market Analysis
11.1 Key Findings
11.2 Latin America Orphan Drugs Market Attractiveness Index
11.3 Latin America Orphan Drugs Market by Country, 2023, 2028 & 2033 (US$ Billion)
11.4 Latin America Orphan Drugs Market Size Estimation and Forecast by Country
11.5 Latin America Orphan Drugs Market Size Estimation and Forecast by Therapeutic Area
11.6 Latin America Orphan Drugs Market Size Estimation and Forecast by Drug Type
11.7 Brazil Orphan Drugs Market Analysis
11.8 Mexico Orphan Drugs Market Analysis
11.9 Argentina Orphan Drugs Market Analysis
11.10 Colombia Orphan Drugs Market Analysis
11.11 Rest of Latin America Orphan Drugs Market Analysis
12 MEA Orphan Drugs Market Analysis
12.1 Key Findings
12.2 MEA Orphan Drugs Market Attractiveness Index
12.3 MEA Orphan Drugs Market by Country, 2023, 2028 & 2033 (US$ Billion)
12.4 MEA Orphan Drugs Market Size Estimation and Forecast by Country
12.5 MEA Orphan Drugs Market Size Estimation and Forecast by Therapeutic Area
12.6 MEA Orphan Drugs Market Size Estimation and Forecast by Drug Type
12.7 GCC Orphan Drugs Market Analysis
12.8 South Africa Orphan Drugs Market Analysis
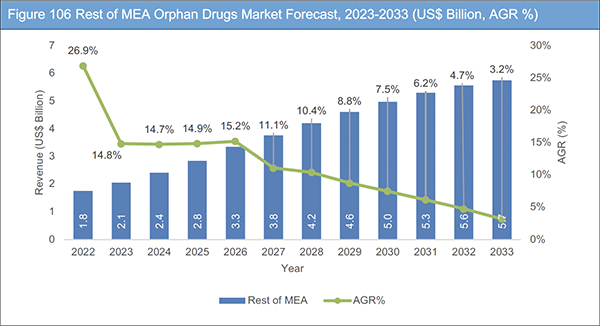
12.9 Rest of MEA Orphan Drugs Market Analysis
13 Company Profiles
13.1 Competitive Landscape, 2022
13.2 Strategic Outlook
13.3 Merck & Co., Inc.
13.3.1 Company Snapshot
13.3.2 Company Overview
13.3.3 Financial Analysis
13.3.3.1 Net Revenue, 2017-2022
13.3.3.2 R&D, 2017-2022
13.3.3.3 Regional Market Shares, 2022
13.3.4 Product Benchmarking
13.3.5 Strategic Outlook
13.4 AbbVie Inc.
13.4.1 Company Snapshot
13.4.2 Company Overview
13.4.3 Financial Analysis
13.4.3.1 Net Revenue, 2017-2022
13.4.3.2 R&D, 2017-2022
13.4.3.3 Regional Market Shares, 2022
13.4.4 Product Benchmarking
13.4.5 Strategic Outlook
13.5 Vertex Pharmaceuticals Incorporated
13.5.1 Company Snapshot
13.5.2 Company Overview
13.5.3 Financial Analysis
13.5.3.1 Net Revenue, 2017-2022
13.5.3.2 R&D, 2017-2022
13.5.3.3 Regional Market Shares, 2022
13.5.4 Product Benchmarking
13.5.5 Strategic Outlook
13.6 Pfizer Inc.
13.6.1 Company Snapshot
13.6.2 Company Overview
13.6.3 Financial Analysis
13.6.3.1 Net Revenue, 2017-2022
13.6.3.2 R&D, 2017-2022
13.6.3.3 Regional Market Shares, 2022
13.6.4 Product Benchmarking
13.6.5 Strategic Outlook
13.7 Bristol-Myers Squibb Company
13.7.1 Company Snapshot
13.7.2 Company Overview
13.7.3 Financial Analysis
13.7.3.1 Net Revenue, 2017-2022
13.7.3.2 R&D, 2017-2022
13.7.3.3 Regional Market Shares, 2022
13.7.4 Product Benchmarking
13.7.5 Strategic Outlook
13.8 AstraZeneca
13.8.1 Company Snapshot
13.8.2 Company Overview
13.8.3 Financial Analysis
13.8.3.1 Net Revenue, 2017-2022
13.8.3.2 R&D, 2017-2022
13.8.3.3 Regional Market Shares, 2022
13.8.4 Product Benchmarking
13.8.5 Strategic Outlook
13.9 Johnson & Johnson Services, Inc.
13.9.1 Company Snapshot
13.9.2 Company Overview
13.9.3 Financial Analysis
13.9.3.1 Net Revenue, 2017-2022
13.9.3.2 R&D, 2017-2022
13.9.3.3 Regional Market Shares, 2022
13.9.4 Product Benchmarking
13.9.5 Strategic Outlook
13.10 F. Hoffmann-La Roche Ltd.
13.10.1 Company Snapshot
13.10.2 Company Overview
13.10.3 Financial Analysis
13.10.3.1 Net Revenue, 2017-2022
13.10.3.2 R&D, 2017-2022
13.10.3.3 Regional Market Shares, 2022
13.10.4 Product Benchmarking
13.10.5 Strategic Outlook
13.11 Novartis AG
13.11.1 Company Snapshot
13.11.2 Company Overview
13.11.3 Financial Analysis
13.11.3.1 Net Revenue, 2017-2022
13.11.3.2 R&D, 2017-2022
13.11.3.3 Regional Market Shares, 2022
13.11.4 Product Benchmarking
13.11.5 Strategic Outlook
13.12 Biogen
13.12.1 Company Snapshot
13.12.2 Company Overview
13.12.3 Financial Analysis
13.12.3.1 Net Revenue, 2017-2022
13.12.3.2 R&D, 2017-2022
13.12.3.3 Regional Market Shares, 2022
13.12.4 Product Benchmarking
13.12.5 Strategic Outlook
13.13 GSK plc
13.13.1 Company Snapshot
13.13.2 Company Overview
13.13.3 Financial Analysis
13.13.3.1 Net Revenue, 2017-2022
13.13.3.2 R&D, 2017-2022
13.13.3.3 Regional Market Shares, 2022
13.13.4 Product Benchmarking
13.13.5 Strategic Outlook
13.14 Sanofi
13.14.1 Company Snapshot
13.14.2 Company Overview
13.14.3 Financial Analysis
13.14.3.1 Net Revenue, 2017-2022
13.14.3.2 R&D, 2017-2022
13.14.3.3 Regional Market Shares, 2022
13.14.4 Product Benchmarking
13.14.5 Strategic Outlook
14 Conclusion and Recommendations
14.1 Concluding Remarks from Visiongain
14.2 Recommendations for Market Players
List of Tables
Table 1 Orphan Drugs Market Snapshot, 2024 & 2034 (US$ Billion, CAGR %)
Table 2 Capital Raised by Rare Disease Therapeutics Companies to Date in 2021 (US$ Million)
Table 3 Orphan Drugs Market, by Region, 2023-2033 (US$ Billion, AGR%, CAGR%): "V" Shaped Recovery
Table 4 Orphan Drugs Market, by Region, 2023-2033 (US$ Billion, AGR%, CAGR%): "U" Shaped Recovery
Table 5 Orphan Drugs Market, by Region, 2023-2033 (US$ Billion, AGR%, CAGR%): "W" Shaped Recovery
Table 6 Orphan Drugs Market, by Region, 2023-2033 (US$ Billion, AGR%, CAGR%): "L" Shaped Recovery
Table 7 Orphan Drugs Market Forecast by Therapeutic Area, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 8 Oncology Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 9 Neurological Diseases Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 10 Cardiovascular Diseases Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 11 Hematologic Disorders Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 12 Infectious Diseases Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 13 Metabolic Diseases Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 14 Others Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 15 Orphan Drugs Market Forecast by Drug Type, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 16 Biologics Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 17 Biosimilar Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 18 Others Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 19 Orphan Drugs Market, by Top 10 Brands, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 20 Orphan Drugs Market Forecast by Region, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 21 North America Orphan Drugs Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 22 North America Orphan Drugs Market Forecast by Therapeutic Area, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 23 North America Orphan Drugs Market Forecast by Drug Type, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 24 U.S Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 25 Canada Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 26 Europe Orphan Drugs Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 27 Europe Orphan Drugs Market Forecast by Therapeutic Area, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 28 Europe Orphan Drugs Market Forecast by Drug Type, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 29 Germany Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 30 France Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 31 UK Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 32 Italy Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 33 Spain Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 34 Russia Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 35 Rest of Europe Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 36 Asia Pacific Orphan Drugs Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 37 Asia Pacific Orphan Drugs Market Forecast by Therapeutic Area, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 38 Asia Pacific Orphan Drugs Market Forecast by Drug Type, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 39 Japan Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 40 China Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 41 India Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 42 Australia Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 43 South Korea Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 44 Singapore Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 45 Rest of Asia Pacific Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 46 Latin America Orphan Drugs Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 47 Latin America Orphan Drugs Market Forecast by Therapeutic Area, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 48 Latin America Orphan Drugs Market Forecast by Drug Type, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 49 Brazil Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 50 Mexico Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 51 Argentina Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 52 Colombia Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 53 Rest of Latin America Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 54 MEA Orphan Drugs Market Forecast by Country, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 55 MEA Orphan Drugs Market Forecast by Therapeutic Area, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 56 MEA Orphan Drugs Market Forecast by Drug Type, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 57 GCC Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 58 South Africa Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 59 Rest of MEA Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR%, CAGR%)
Table 60 Strategic Outlook
Table 61 Merck & Co., Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 62 Merck & Co., Inc.: Product Benchmarking
Table 63 Merck & Co., Inc.: Strategic Outlook
Table 64 AbbVie Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 65 AbbVie Inc.: Product Benchmarking
Table 66 AbbVie Inc.: Strategic Outlook
Table 67 Vertex Pharmaceuticals Incorporated: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 68 Vertex Pharmaceuticals Incorporated: Product Benchmarking
Table 69 Vertex Pharmaceuticals Incorporated: Strategic Outlook
Table 70 Pfizer Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 71 Pfizer Inc.: Product Benchmarking
Table 72 Pfizer Inc.: Strategic Outlook
Table 73 Bristol-Myers Squibb Company: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 74 Bristol-Myers Squibb Company: Product Benchmarking
Table 75 Bristol-Myers Squibb Company: Strategic Outlook
Table 76 AstraZeneca: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 77 AstraZeneca: Product Benchmarking
Table 78 AstraZeneca: Strategic Outlook
Table 79 Johnson & Johnson Services, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 80 Johnson & Johnson Services, Inc.: Product Benchmarking
Table 81 Johnson & Johnson Services, Inc.: Strategic Outlook
Table 82 F. Hoffmann-La Roche Ltd.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 83 F. Hoffmann-La Roche Ltd.: Product Benchmarking
Table 84 F. Hoffmann-La Roche Ltd.: Strategic Outlook
Table 85 Novartis AG: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 86 Novartis AG: Product Benchmarking
Table 87 Novartis AG: Strategic Outlook
Table 88 Biogen: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 89 Biogen: Product Benchmarking
Table 90 Biogen: Strategic Outlook
Table 91 GSK plc: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 92 GSK plc: Product Benchmarking
Table 93 GSK plc: Strategic Outlook
Table 94 Sanofi: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 95 Sanofi: Product Benchmarking
Table 96 Sanofi: Strategic Outlook
List of Figures
Figure 1 Orphan Drugs Market Segmentation
Figure 2 Orphan Drugs Market by Therapeutic Area: Market Attractiveness Index
Figure 3 Orphan Drugs Market by Drug Type: Market Attractiveness Index
Figure 4 Orphan Drugs Market by Brand: Market Attractiveness Index
Figure 5 Orphan Drugs Market Attractiveness Index by Region
Figure 6 Orphan Drugs Market: Market Dynamics
Figure 7 Orphan Drugs Market by Region, 2023-2033 (US$ Billion, AGR %): “V” Shaped Recovery
Figure 8 Orphan Drugs Market by Region, 2023-2033 (US$ Billion, AGR %): “U” Shaped Recovery
Figure 9 Orphan Drugs Market by Region, 2023-2033 (US$ Billion, AGR %): “W” Shaped Recovery
Figure 10 Orphan Drugs Market by Region, 2023-2033 (US$ Billion, AGR %): “L” Shaped Recovery
Figure 11 Orphan Drugs Market: Porter’s Five Forces Analysis
Figure 12 Orphan Drugs Market: PEST Analysis
Figure 13 Orphan Drugs Market by Therapeutic Area: Market Attractiveness Index
Figure 14 Orphan Drugs Market Forecast by Therapeutic Area, 2023-2033 (US$ Billion, AGR %)
Figure 15 Orphan Drugs Market Share Forecast by Therapeutic Area, 2023, 2028, 2033 (%)
Figure 16 Oncology Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR %)
Figure 17 Oncology Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 18 Neurological Diseases Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR %)
Figure 19 Neurological Diseases Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 20 Cardiovascular Diseases Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR %)
Figure 21 Cardiovascular Diseases Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 22 Hematologic Disorders Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR %)
Figure 23 Hematologic Disorders Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 24 Infectious Diseases Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR %)
Figure 25 Infectious Diseases Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 26 Metabolic Diseases Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR %)
Figure 27 Metabolic Diseases Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 28 Others Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR %)
Figure 29 Others Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 30 Orphan Drugs Market by Drug Type: Market Attractiveness Index
Figure 31 Orphan Drugs Market Forecast by Drug Type, 2023-2033 (US$ Billion, AGR %)
Figure 32 Orphan Drugs Market Share Forecast by Drug Type, 2023, 2028, 2033 (%)
Figure 33 Biologics Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR %)
Figure 34 Biologics Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 35 Biosimilar Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR %)
Figure 36 Biosimilar Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 37 Others Segment Market Forecast by Region, 2023-2033 (US$ Billion, AGR %)
Figure 38 Others Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 39 Orphan Drugs Market Forecast by Top 10 brands, 2023-2033 (US$ Billion, AGR %)
Figure 40 Orphan Drugs Market Forecast by Region 2023 and 2033 (Revenue, CAGR%)
Figure 41 Orphan Drugs Market Share Forecast by Region 2023, 2028, 2033 (%)
Figure 42 Orphan Drugs Market by Region, 2023-2033 (US$ Billion, AGR %)
Figure 43 North America Orphan Drugs Market Attractiveness Index
Figure 44 North America Orphan Drugs Market by Region, 2023, 2028 & 2033 (US$ Billion)
Figure 45 North America Orphan Drugs Market Forecast by Country, 2023-2033 (US$ Billion, AGR %)
Figure 46 North America Orphan Drugs Market Share Forecast by Country, 2023 & 2033 (%)
Figure 47 North America Orphan Drugs Market Forecast by Therapeutic Area, 2023-2033 (US$ Billion, AGR %)
Figure 48 North America Orphan Drugs Market Share Forecast by Therapeutic Area, 2023 & 2033 (%)
Figure 49 North America Orphan Drugs Market Forecast by Drug Type, 2023-2033 (US$ Billion, AGR %)
Figure 50 North America Orphan Drugs Market Share Forecast by Drug Type, 2023 & 2033 (%)
Figure 51 U.S. Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 52 Canada Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 53 Europe Orphan Drugs Market Attractiveness Index
Figure 54 Europe Orphan Drugs Market by Region, 2023, 2028 & 2033 (US$ Billion)
Figure 55 Europe Orphan Drugs Market Forecast by Country, 2023-2033 (US$ Billion, AGR %)
Figure 56 Europe Orphan Drugs Market Share Forecast by Country, 2023 & 2033 (%)
Figure 57 Europe Orphan Drugs Market Forecast by Therapeutic Area, 2023-2033 (US$ Billion, AGR %)
Figure 58 Europe Orphan Drugs Market Share Forecast by Therapeutic Area, 2023 & 2033 (%)
Figure 59 Europe Orphan Drugs Market Forecast by Drug Type, 2023-2033 (US$ Billion, AGR %)
Figure 60 Europe Orphan Drugs Market Share Forecast by Drug Type, 2023 & 2033 (%)
Figure 61 Germany Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 62 France Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 63 UK Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 64 Italy Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 65 Spain Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 66 Russia Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 67 Rest of Europe Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 68 Asia Pacific Orphan Drugs Market Attractiveness Index
Figure 69 Asia Pacific Orphan Drugs Market by Region, 2023, 2028 & 2033 (US$ Billion)
Figure 70 Asia Pacific Orphan Drugs Market Forecast by Country, 2023-2033 (US$ Billion, AGR %)
Figure 71 Asia Pacific Orphan Drugs Market Share Forecast by Country, 2023 & 2033 (%)
Figure 72 Asia Pacific Orphan Drugs Market Forecast by Therapeutic Area, 2023-2033 (US$ Billion, AGR %)
Figure 73 Asia Pacific Orphan Drugs Market Share Forecast by Therapeutic Area, 2023 & 2033 (%)
Figure 74 Asia Pacific Orphan Drugs Market Forecast by Drug Type, 2023-2033 (US$ Billion, AGR %)
Figure 75 Asia Pacific Orphan Drugs Market Share Forecast by Drug Type, 2023 & 2033 (%)
Figure 76 Japan Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 77 China Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 78 India Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 79 Australia Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 80 South Korea Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 81 Singapore Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 82 Rest of Asia Pacific Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 83 Latin America Orphan Drugs Market Attractiveness Index
Figure 84 Latin America Orphan Drugs Market by Region, 2023, 2028 & 2033 (US$ Billion)
Figure 85 Latin America Orphan Drugs Market Forecast by Country, 2023-2033 (US$ Billion, AGR %)
Figure 86 Latin America Orphan Drugs Market Share Forecast by Country, 2023 & 2033 (%)
Figure 87 Latin America Orphan Drugs Market Forecast by Therapeutic Area, 2023-2033 (US$ Billion, AGR %)
Figure 88 Latin America Orphan Drugs Market Share Forecast by Therapeutic Area, 2023 & 2033 (%)
Figure 89 Latin America Orphan Drugs Market Forecast by Drug Type, 2023-2033 (US$ Billion, AGR %)
Figure 90 Latin America Orphan Drugs Market Share Forecast by Drug Type, 2023 & 2033 (%)
Figure 91 Brazil Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 92 Mexico Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 93 Argentina Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 94 Colombia Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 95 Rest of Latin America Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 96 MEA Orphan Drugs Market Attractiveness Index
Figure 97 MEA Orphan Drugs Market by Region, 2023, 2028 & 2033 (US$ Billion)
Figure 98 MEA Orphan Drugs Market Forecast by Country, 2023-2033 (US$ Billion, AGR %)
Figure 99 MEA Orphan Drugs Market Share Forecast by Country, 2023 & 2033 (%)
Figure 100 MEA Orphan Drugs Market Forecast by Therapeutic Area, 2023-2033 (US$ Billion, AGR %)
Figure 101 MEA Orphan Drugs Market Share Forecast by Therapeutic Area, 2023 & 2033 (%)
Figure 102 MEA Orphan Drugs Market Forecast by Drug Type, 2023-2033 (US$ Billion, AGR %)
Figure 103 MEA Orphan Drugs Market Share Forecast by Drug Type, 2023 & 2033 (%)
Figure 104 GCC Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 105 South Africa Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 106 Rest of MEA Orphan Drugs Market Forecast, 2023-2033 (US$ Billion, AGR %)
Figure 107 Orphan Drugs Market: Company Share/Ranking, 2022
Figure 108 Merck & Co., Inc.: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 109 Merck & Co., Inc.: R&D, 2017-2022 (US$ Million, AGR%)
Figure 110 Merck & Co., Inc.: Regional Market Shares, 2022
Figure 111 AbbVie Inc.: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 112 AbbVie Inc.: R&D, 2017-2022 (US$ Million, AGR%)
Figure 113 AbbVie Inc.: Regional Market Shares, 2022
Figure 114 Vertex Pharmaceuticals Incorporated: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 115 Vertex Pharmaceuticals Incorporated: R&D, 2017-2022 (US$ Million, AGR%)
Figure 116 Vertex Pharmaceuticals Incorporated: Regional Market Shares, 2022
Figure 117 Pfizer Inc.: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 118 Pfizer Inc.: R&D, 2017-2022 (US$ Million, AGR%)
Figure 119 Pfizer Inc.: Regional Market Shares, 2022
Figure 120 Bristol-Myers Squibb Company: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 121 Bristol-Myers Squibb Company: R&D, 2017-2022 (US$ Million, AGR%)
Figure 122 Bristol-Myers Squibb Company: Regional Market Shares, 2022
Figure 123 AstraZeneca: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 124 AstraZeneca: R&D, 2017-2022 (US$ Million, AGR%)
Figure 125 AstraZeneca: Regional Market Shares, 2022
Figure 126 Johnson & Johnson Services, Inc.: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 127 Johnson & Johnson Services, Inc.: R&D, 2017-2022 (US$ Million, AGR%)
Figure 128 Johnson & Johnson Services, Inc.: Regional Market Shares, 2022
Figure 129 F. Hoffmann-La Roche Ltd.: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 130 F. Hoffmann-La Roche Ltd.: R&D, 2017-2022 (US$ Million, AGR%)
Figure 131 F. Hoffmann-La Roche Ltd.: Regional Market Shares, 2022
Figure 132 Novartis AG: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 133 Novartis AG: R&D, 2017-2022 (US$ Million, AGR%)
Figure 134 Novartis AG: Regional Market Shares, 2022
Figure 135 Biogen: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 136 Biogen: R&D, 2017-2022 (US$ Million, AGR%)
Figure 137 Biogen: Regional Market Shares, 2022
Figure 138 GSK plc: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 139 GSK plc: R&D, 2017-2022 (US$ Million, AGR%)
Figure 140 GSK plc: Regional Market Shares, 2022
Figure 141 Sanofi: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 142 Sanofi: R&D, 2017-2022 (US$ Million, AGR%)
Figure 143 Sanofi: Regional Market Shares, 2022
List of Companies Profiled in the Report
AbbVie Inc.
AstraZeneca
Biogen
Bristol-Myers Squibb Company
F. Hoffmann-La Roche Ltd.
GSK plc
Johnson & Johnson Services, Inc.
Merck & Co., Inc.
Novartis AG
Pfizer Inc.
Sanofi
Vertex Pharmaceuticals Incorporated
List of Other Companies Mentioned in the Report
2seventy bio, Inc.
Alexion
Amgen Inc.
Beam Therapeutics
CRISPR Therapeutics
DJS Antibodies
Incyte
Lilly
Neogene Therapeutics Inc.
Pharmacyclics
List of Associations Mentioned in the Report
European Medicines Agency (EMA)
Indian Council of Medical Research (ICMR)
Italian Medicines Agency (AIFA)
Ministry of Food and Drug Safety (MFDS)
National Organization for Rare Disorders (NORD)
The Canadian Agency for Drugs and Technologies in Health (CADTH)
Spain's National Health System (Sistema Nacional de Salud; SNS)
National Institute of Drug and Food Surveillance (INVIMA)
U.S. Food and Drug Administration (FDA)






















