Industries > Pharma > Needle-Free Delivery Technology Market 2018-2028
Needle-Free Delivery Technology Market 2018-2028
Jet Injector Technology, Competing Technologies, Novel Needle Technology, Inhaler Technology, Patch Technology
The global needle-free delivery technology market was valued at $1.7bn in 2017 and is estimated to reach $5.5bn by 2028, growing at a CAGR of 11.5% from 2018-2028. In 2017, the Jet Injectors submarket held 45.1% of the global needle-free delivery technology market.
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in this sector.
In this brand new 183-page report you will receive 107 charts– all unavailable elsewhere.
The 183-page report provides clear detailed insight into the global needle-free delivery technology market. Discover the key drivers and challenges affecting the market.
By ordering and reading our brand new report today you stay better informed and ready to act.
Report Scope
• Global Needle-Free Delivery Technology market forecasts from 2018-2028
• Jet Injector submarket forecasts from 2018-2028
• Competing Technology submarket forecast from 2018-2028, further segmented into:
• Novel Needle Technology
• Inhaler Technology
• Patch Technology
• This report also breaks down the revenue forecast for global needle-free delivery technology market by regional and national market:
• The US
• EU5: Germany, France, UK, Italy, Spain
• Japan
• China
• India
• This report discusses the leading companies that are involved in the needle-free delivery technology market. It contains overviews of the companies’ activities, strategies and recent financial results:
• 3M
• Antares Pharma
• Becton Dickinson
• BioJect Medical Technologies
• PharmaJet
• Zogenix
• This report provides a SWOT Analysis of the global needle-free delivery technology market.
Visiongain’s study is for everybody needing commercial analyses for the needle-free delivery technology market and leading companies. You will find data, trends and predictions. Please order our report now.
Buy our report today Needle-Free Delivery Technology Market Forecast 2018-2028: Jet Injector Technology, Competing Technologies, Novel Needle Technology, Inhaler Technology, Patch Technology.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
1.1 Global Needle-Free Delivery Technology Market Overview
1.2 Global Needle-Free Delivery Technology Market Segmentation
1.3 Why You Should Read This Report
1.4 How This Report Delivers
1.5 Key Questions Answered by This Analytical Report
1.6 Who is This Report For?
1.7 Methodology
1.8 Frequently Asked Questions (FAQ)
1.9 Associated Visiongain Reports
1.10 About Visiongain
2. Introduction to Needle-Free Delivery Technology
2.1 What is Needle-Free Delivery Technology?
2.1.1 Needlestick Injury
2.1.1.1 Blood-Borne Pathogens and Needlestick Injury
2.1.1.2 Accidental Needlestick Injury: A Serious Healthcare Problem
2.2 The Needle-Free Delivery Technology Market
2.2.1 Jet Injectors
2.2.1.1 Concerns about Multi-use Nozzle Jet injectors (MUNJIs)
2.2.1.2 Strengths and Weaknesses of Jet Injectors
2.2.2 Competing Needle-Free Technologies
2.2.2.1 Novel Needle Technology
2.2.2.2 Pen Needles
2.2.2.3 Microneedles
2.2.2.4 Hollow Microneedles
2.2.2.5 Solid Coated Microneedles
2.2.2.6 Solid Biodegradable Microneedles
2.2.2.7 Solid Uncoated Microneedles
2.2.2.8 Using the Right Microneedles
2.2.2.9 Inhaler Technology
2.2.2.10 Transdermal Patch Technology
2.3 Clinical Settings Where Needle-Free Delivery Technology Will Be Beneficial
2.3.1 Pain Management
2.3.1.1 Using Needle-Free Injection Devices to Administer Lidocaine
2.3.2 Vaccine Delivery: Improving Immune Response
2.3.2.1 Needle-Free Vaccine Delivery
2.3.2.2 Mass Immunisation
2.3.3 Insulin Delivery for Diabetics
2.3.4 Paediatric Injections
2.3.5 Other Uses for Needle-Free Injection Technology
2.4 Regulation of the Needle-Free Delivery Technology Market
2.4.1 The US Regulation System
2.4.1.1 Combination Products
2.4.1.2 FDA Statement Clamps Down On Delivery of Medications in Non-Approved Needle-Free Devices
2.4.2 The European Regulation System
2.4.2.1 Post Marketing Surveillance in the EU
2.5 Biopharmaceuticals
2.5.1 An Introduction to the Biological Drugs Market
2.5.2 Biologics: A Rising Drugs Market
3. Needle-Free Delivery Technology: Global Market 2018-2028
3.1 The Needle-Free Delivery Technology Market: Overview
3.2 Needle-Free Delivery Technology Market Segmentation, 2017
3.3 The Needle-Free Delivery Technology Market Forecast, 2017-2028
3.4 How Will Segmental Market Shares Change to 2028?
3.5 Needle-Free Delivery Technology: Market Trends, 2018-2028
3.6 Needle-Free Delivery Technology: Drivers and Restraints 2018-2028
4. Jet Injectors Technology Market, 2018-2028
4.1 The Jet Injectors Market Overview
4.2 Jet Injectors: Market Forecast 2017-2028
4.3 Jet Injector Devices on the Market
4.3.1 Biojector 2000 (Bioject Medical Technologies)
4.3.2 ZetaJet (Bioject Medical Technologies)
4.3.3 Vision (Antares Pharma)
4.3.4 Vibex (Antares Pharma)
4.3.5 Sumavel DosePro (Endo International)
4.3.6 Stratis (PharmaJet)
4.3.7 LectraJet (D’Antonio Consultants International)
4.3.8 AdvantaJet and GentleJet (Activa Brand Products)
4.3.9 Vitajet (Bioject Medical Technologies)
4.3.10 Med-Jet (Medical International Technologies)
4.3.11 J-Tip (National Medical Products)
4.3.12 Injex30 (Injex Pharma)
4.3.13 SQ-Pen, SQ-X and MHP-1 (Bespak)
4.3.14 E-Jet 100 (Eurojet Medical)
4.3.15 Penjet (Penjet Corporation)
4.4 Jet Injectors Development Pipeline
4.4.1 Glide SDI (Glide Pharmaceutical Technologies)
4.4.2 Zeneo (Crossject)
4.4.3 Relday (Zogenix)
4.4.4 Magnetic Injection (Massachusetts Institute of Technology)
4.4.5 Intradermal (ID) Pen (Bioject Medical Technologies)
4.4.6 Iject and Iject R (Bioject Medical Technologies)
4.4.7 Jupiter Jet (Bioject Medical Technologies)
5. Competing Needle-Free Technology Market, 2018-2028
5.1 The Competing Needle-Free Technology Overview
5.2 The Competing Technology: Market Forecast 2017-2028
5.3 How Will Segmental Market Shares Change to 2028?
5.4 Novel Needle Technology Overview
5.5 Novel Needle Technology: Market Forecast, 2018-2028
5.5.1 Leading Novel Needle Technologies
5.5.1.1 Soluvia Microinjection System (BD)
5.5.1.2 Ultra-Fine Nano 4mm Pen Needle (BD)
5.5.1.3 AutoShield Duo Pen Needle (BD)
5.5.1.4 Microstructured Transdermal Systems (3M)
5.5.1.5 ZP Patch Technology (Zosano)
5.5.2 Novel Needle Technology Development Pipeline
5.5.2.1 Microneedle Patch (Georgia Institute of Technology)
5.5.2.2 Micronjet (NanoPass)
5.5.2.3 DebioJect (Debiotech)
5.5.2.4 AdminPen (nanoBioSciences)
5.5.2.5 PKA SoftTouch (PKA SoftTouch Corp)
5.5.2.6 DrugMat and VaxMat (TheraJect)
5.5.2.7 MicroCor (Corium International)
5.5.2.8 Nanopatch (Vaxxas)
5.5.2.9 Nanotopography Enhanced Microneedle Delivery (University of California-San Francisco, Georgia Institute of Technology and Kimberly-Clark)
5.5.2.10 Memspatch and Micro-Patch (Nemaura)
5.5.2.11 Microneedle Patch for Lidocaine Delivery (National University of Singapore)
5.6 Inhaler Technology Overview
5.7 Inhaler Technology: Market Forecast, 2017-2028
5.7.1 Inhaler Products in the Asthma Market
5.7.1.1 Preventer Inhalers
5.7.1.2 Reliever Inhalers
5.7.2 Inhaler Products in the Insulin Market
5.7.3 Inhaler Products in the Vaccine Market: FluMist Quadrivalent (MedImmune)
5.7.3.1 Pipeline
5.8 Transdermal Patch Technology Overview
5.9 Transdermal Patch Technology: Market Forecast, 2017-2028
5.9.1 Selected Transdermal Patch Products
5.9.1.1 Duragesic (Fentanyl, Janssen Pharmaceuticals)
5.9.1.2 Nicoderm CQ (Nicotine, GlaxoSmithKline)
5.9.1.3 Exelon Patch (Rivastigmine, Novartis)
5.9.1.4 Zecuity (Sumatriptan, Teva)
5.9.2 Transdermal Patch Technology Development Pipeline
5.9.2.1 Electronic Transdermal Patch (Rhenovia Pharma)
5.9.2.2 Electroporation-Mediated DNA Drug Delivery (TriGrid, Ichor Medical Systems)
5.9.2.3 Ultrasonic Waveforms (U-Strip, Transdermal Specialties)
5.9.2.4 Prelude SkinPrep System (Echo Therapeutics)
5.10 Other Competing Needle-free delivery Technology in development
5.10.1 Microbubbles Injection (Shibaura Institute of Technology)
5.10.2 Laser Injection (Seoul National University)
5.10.3 Vaccine Delivery Using Micro-Shock Waves (Indian Institute of Science, Bangalore)
5.10.4 Nanotechnology Syringes (Gwangju Institute of Science and Technology, South Korea)
6. Leading National Markets 2017-2028
6.1 Global Needle-Free Delivery Technology Market: Regional Forecast 2018-2028
6.1.1 How Will Regional Market Shares Change to 2026?
6.2 The US Needle-Free Delivery Technology Market 2018-2028
6.2.1 US Needle-Free Delivery Technology Market Forecast 2018-2028
6.2.2 FDA Guideline for Combination Products
6.2.3 Needle-Free Delivery and the US Flu Vaccination Market
6.2.4 Measles Outbreak in the US
6.3 The EU5 Needle-Free Delivery Technology Market 2017-2028
6.3.1 EU5 Needle-Free Delivery Technology Market Forecast 2018-2028
6.3.2 Germany Needle-Free Delivery Technology Market Forecast, 2018-2028
6.3.3 France Needle-Free Delivery Technology Market Forecast, 2018-2028
6.3.4 The UK Needle-Free Delivery Technology Market Forecast, 2018-2028
6.3.5 Italy Needle-Free Delivery Technology Market Forecast, 2018-2028
6.3.6 Spain Needle-Free Delivery Technology Market Forecast, 2018-2028
6.4 Japan Needle-Free Delivery Technology Market 2018-2028
6.4.1 Japan Needle-Free Delivery Technology Market Forecast 2018-2028
6.5 China Needle-Free Delivery Technology Market 2018-2028
6.5.1 China Needle-Free Delivery Technology Market Forecast 2018-2028
6.6 India Needle-Free Delivery Technology Market 2018-2028
6.6.1 India Needle-Free Delivery Technology Market Forecast 2018-2028
6.6.2 Needles and Syringes being Re-used in Indian Hospitals
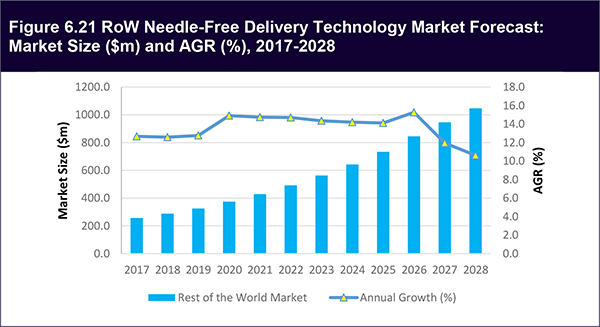
6.7 The Rest of the World (RoW) Needle-Free Delivery Technology Market 2018-2028
6.7.1 RoW Needle-Free Delivery Technology Market Forecast 2018-2028
7. Leading Companies in the Needle-Free Delivery Technology Market
7.1 Leading Needle-Free Delivery Technology Manufacturers in 2017
7.2 Antares Pharma: Leading the Needle-Free Injector Market
7.2.1 Antares Pharma: Needle-Free Technology Product Portfolio
7.2.2 Antares Pharma: Future Outlook
7.2.3 Antares Pharma: Products in Development
7.2.4 Settlement of Patent Infringement Case between Antares Pharma and Medac Pharma
7.3 Zogenix
7.3.1 Zogenix: Product Portfolio
7.3.2 Zogenix: Current Pipeline
7.3.3 Zogenix: Financial Overview
7.4 3M: 50 Years of Drug Delivery Technology Development
7.4.1 3M: Drug Delivery Technology Portfolio
7.4.1.1 Oral and Topical Drug Delivery
7.4.1.2 MDI for Nasal and Inhalable Drugs
7.4.1.3 Transdermal patch Technology
7.4.1.4 Microneedle Technology
7.4.2 3M: Market Outlook
7.5 Becton Dickinson and Company (BD)
7.5.1 BD: Drug Delivery Technology Portfolio
7.5.1.1 Prefillable Syringe Systems
7.5.1.2 Self-Injection Systems
7.5.1.3 Pen Needles
7.5.1.4 Safety & Shielding Systems
7.5.2 BD: Future Outlook
7.5.3 New Infusion Set with BD FlowSmart Technology Cleared by FDA
7.5.4 BD Acquired Injection Safety Firms
7.5.5 Recall of BD Needles in Hong Kong
7.6 PharmaJet
7.6.1 PharmaJet: Needle-Free Delivery Technology Product Portfolio
7.6.2 First FDA Approved Jet Injector for Flu Shot in the US
7.6.3 PharmaJet: Collaborations and Needle-Free Delivery Technology Pipeline
7.6.4 Preference for PharmaJet over Needle and Syringe
7.7 BioJect Medical Technologies
7.7.1 Bioject Medical Technologies: Needle-Free Delivery Technology Product Portfolio and Pipeline
7.7.2 Bioject Medical Technologies: Financial Overview
7.7.3 Focusing on Developing Countries
8. Qualitative Analysis of the Needle-Free Delivery Technology Market 2017-2028
8.1 SWOT Analysis of the Needle-Free Delivery Technology Market
8.2 Strengths
8.2.1 Strong Growth in the Biologic Drug Market
8.2.2 Increasing Development in Transdermal Delivery
8.2.3 No Specialist Training Required and No Needlestick Injuries
8.2.4 Eliminating the Cold Chain Problem
8.3 Weaknesses
8.3.1 Healthcare Practitioners are not familiar with Novel Delivery Systems
8.3.2 Greater Regulatory Scrutiny
8.3.3 Inefficient Manufacturing Processes
8.4 Opportunities
8.4.1 Emerging Economies Offer Significant Growth Opportunities
8.4.2 Mass Immunisation Programmes around the World
8.4.3 Increasing Global Diabetic Population
8.4.4 Shift towards Home Administration Setting
8.4.5 Extending Life Cycle of Drugs
8.5 Threats
8.5.1 Traditional Needles and Syringes are Very Cheap to Mass Produce
8.5.2 Needle and Syringes with Anti Needlestick Injury Technology
8.5.3 Limited Clinical Data
8.5.4 Medical Device Excise Tax
9. Conclusions
9.1 Current Leading Needle-Free Technology Segments
9.1.1 Notable Needle-Free Technology Companies
9.1.2 Leading Regional Markets
9.2 Global Needle-Free Technology Market Forecast 2018-2028
9.3 The Future of the Needle-Free Technology Market
9.3.1 Biosimilars are a Major Driver of the Needle-free Delivery Market
9.3.2 Strong Pipeline Will Drive the Competing Needle-Free Injection Technology Market
Appendices
Glossary
Associated Visiongain Reports
Visiongain Report Sales Order Form
Appendix A
About Visiongain
Appendix B
Visiongain report evaluation form
List of Tables
Table 2.1 Classification of Jet Injectors by Method of Propulsion
Table 2.2 Strengths and Weaknesses of the Jet Injector Market
Table 2.3 Pen Needle Technology by Needle Length
Table 2.4 Rationale for Needle-Free Vaccine Delivery
Table 2.5 Examples of Needle-Free Vaccine Delivery Systems, 2017
Table 2.6 Examples of Needle-Free Insulin Delivery Systems for Diabetics, 2017
Table 2.7 FDA Guidance on Regulation of Needle-Free Injectors, 2017
Table 2.8 Top 5 Biologics by Sales ($bn) in U.S, 2017
Table 3.1 The Needle-Free Delivery Technology Market: Revenue ($m), Market Share (%) by Segment, 2017
Table 3.2 The Needle-Free Delivery Technology Market: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 3.3 Needle-Free Delivery Market Segments: Market Size ($m) and Market Share (%), 2017, 2022 and 2028
Table 4.1 The Jet Injectors Market: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 5.1 The Competing Needle-Free Technology Market: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 5.2 Market Size ($m) and Market Share (%) of Competing Needle-Free Technology Market Segments, 2017
Table 5.3 Novel Needle Technology Market: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 5.4 Inhaler Technology Market: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 5.5 Leading Products in the Asthma Preventer Inhaler Market, 2017
Table 5.6 Leading Products in the Asthma Reliever Inhaler Market, 2017
Table 5.7 Vaccine Inhalers Currently Under Development, 2017
Table 5.8 Transdermal Patch Technology Market: Revenue ($m), AGR (%) and CAGR (%), 2017-2028
Table 5.9 Transdermal Patch Products, 2017
Table 6.1 Global Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%) and CAGR (%) by Region, 2017-2028
Table 6.2 Global Needle-Free Delivery Technology Market: Market Share (%) by Country/Region, 2017-2028
Table 6.3 Global Needle-Free Delivery Technology Market: Market Size ($m) and Market Share (%) by Country/Region, 2017, 2022 and 2028
Table 6.4 US Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.5 EU5 Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.6 EU5 Needle-Free Delivery Technology Market: Market Shares (%) by National Market, 2017, 2022 and 2028
Table 6.7 Germany Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.8 France Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.9 UK Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%), CAGR (%), 2017-2028
Table 6.10 Italy Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.11 Spain Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.12 Japan Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.13 China Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%), CAGR (%), 2017-2028
Table 6.14 India Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%) and CAGR (%), 2017-2028
Table 6.15 RoW Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%) and CAGR (%), 2017-2028
Table 7.1 Antares Pharma: Overview, 2017
Table 7.2 Antares Pharma: 2017 Product Portfolio (Product, Drug, Partner, Indication and Territory
Table 7.3 Antares Revenue from Major Customers: Revenue ($m) and Revenue Share (%), 2016 and 2017
Table 7.4 Antares Revenue by Region: Revenue ($m) and Revenue Share (%), 2016 and 2017
Table 7.5 Antares Pharma: Historical Revenue ($m), AGR (%) and CAGR (%), 2013-2017
Table 7.6 Antares Pharma: Products in Development 2017 (Product Name, Drug, Partner, Indication, Territory and Regulatory Status)
Table 7.7 Zogenix: Overview, 2017
Table 7.8 Zogenix: Revenue ($m), AGR (%) and CAGR (%), 2013-2017
Table 7.9 Zogenix: Revenue ($m) by Type, 2016 & 2017
Table 7.10 3M: Overview, 2017
Table 7.11 3M: Health Care Historical Revenue ($bn), AGR (%) and CAGR (%), 2013-2017
Table 7.12 BD: Overview, 2017
Table 7.13 BD: Drug Delivery Products by Segment, 2017
Table 7.14 BD: Historical Revenue ($bn) by Geographic Region, 2013-2017
Table 7.15 BD Medical: Historical Revenue ($bn), AGR (%) and CAGR (%), 2013-2017
Table 7.16 PharmaJet: Overview, 2017
Table 7.17 BioJect Medical Technologies: Overview, 2017
Table 7.18 Bioject Medical Technologies: Revenue ($m) by Product Line, 2010
Table 7.19 Bioject Medical Technologies: Revenue ($m), AGR (%) and CAGR (%), 2010-2014
Table 8.1 SWOT Analysis of the Needle-Free Delivery Technology Market, 2017-2028
Table 8.2 Average Price for Needle-Free Drug/Vaccine Delivery Technology, 2017
List of Figures
Figure 1.1 Global Needle-Free Delivery Technology Market Segmentation Overview, 2017
Figure 2.1 Percentage (%) of Needlestick Injuries during the Timeline of Use to Disposal of a Device
Figure 3.1 The Needle-Free Delivery Technology Market: Revenue ($m), AGR (%), 2017-2028
Figure 3.2 Market Shares (%) of Leading Needle-Free Delivery Market Segments, 2017
Figure 3.3 Market Shares (%) of Leading Needle-Free Delivery Market Segments, 2022
Figure 3.4 Market Shares (%) of Leading Needle-Free Delivery Market Segments, 2028
Figure 3.5 Needle-Free Delivery Technology: Drivers and Restraints
Figure 4.1 The Jet Injectors Market: Revenue ($m) and AGR (%), 2017-2028
Figure 5.1 The Competing Needle-Free Technology Market: Revenue ($m) and AGR (%), 2017-2028
Figure 5.2 Market Shares (%) of Competing Needle-Free Technology Market Segments, 2017
Figure 5.3 Market Shares (%) of Leading Needle-Free Delivery Market Segments, 2022
Figure 5.4 Market Shares (%) of Leading Needle-Free Delivery Market Segments, 2028
Figure 5.5 Novel Needle Technology Market: Revenue ($m) and AGR (%), 2017-2028
Figure 5.6 Inhaler Technology Market: Revenue ($m), AGR (%), 2017-2028
Figure 5.7 Transdermal Patch Technology Market: Revenue ($m) and AGR (%), 2017-2028
Figure 6.1 Global Needle-Free Delivery Technology Market: Market Size ($m) and Market Share (%) by Country/Region, 2017, 2022 and 2028
Figure 6.2 Global Needle-Free Delivery Technology Market: CAGR (%) by Country/Region, 2017-2022
Figure 6.3 Global Needle-Free Delivery Technology Market: CAGR (%) by Country/Region, 2022-2028
Figure 6.4 Global Needle-Free Delivery Technology Market: CAGR (%) by Country/Region, 2017-2028
Figure 6.5 Global Needle-Free Delivery Technology Market: Market Share (%) by Country/Region, 2017
Figure 6.6 Global Needle-Free Delivery Technology Market: Market Share (%) by Country/Region, 2022
Figure 6.7 Global Needle-Free Delivery Technology Market: Market Share (%) by Country/Region, 2028
Figure 6.8 US Needle-Free Delivery Technology Market Forecast: Market Size ($m) and AGR (%), 2017-2028
Figure 6.9 EU5 Needle-Free Delivery Technology Market Forecast: Market Size ($m), AGR (%), 2017-2028
Figure 6.10 EU5 Needle-Free Delivery Technology Market: Market Shares (%) by National Market, 2017
Figure 6.11 EU5 Needle-Free Delivery Technology Market: Market Shares (%) by National Market, 2022
Figure 6.12 EU5 Needle-Free Delivery Technology Market: Market Shares (%) by National Market, 2028
Figure 6.13 Germany Needle-Free Delivery Technology Market Forecast: Market Size ($m) and AGR (%), 2017-2028
Figure 6.14 France Needle-Free Delivery Technology Market Forecast: Market Size ($m) and AGR (%), 2017-2028
Figure 6.15 UK Needle-Free Delivery Technology Market Forecast: Market Size ($m) and AGR (%), 2017-2028
Figure 6.16 Italy Needle-Free Delivery Technology Market Forecast: Market Size ($m) and AGR (%), 2017-2028
Figure 6.17 Spain Needle-Free Delivery Technology Market Forecast: Market Size ($m) and AGR (%), 2017-2028
Figure 6.18 Japan Needle-Free Delivery Technology Market Forecast: Market Size ($m) and AGR (%), 2017-2028
Figure 6.19 China Needle-Free Delivery Technology Market Forecast: Market Size ($m) and AGR (%), 2017-2028
Figure 6.20 India Needle-Free Delivery Technology Market Forecast: Market Size ($m) and AGR (%), 2017-2028
Figure 6.21 RoW Needle-Free Delivery Technology Market Forecast: Market Size ($m) and AGR (%), 2017-2028
Figure 7.1 Antares Revenue from Major Customers: Revenue Share (%), 2016
Figure 7.2 Antares Revenue from Major Customers: Revenue Share (%), 2017
Figure 7.3 Antares Revenue by Region: Revenue Share (%), 2016
Figure 7.4 Antares Revenue by Region: Revenue Share (%), 2017
Figure 7.5 Antares Pharma: Historical Revenue ($m) and AGR (%), 2013-2017
Figure 7.6 Zogenix: Revenue ($m) and AGR (%), 2013-2017
Figure 7.7 Zogenix: Revenue ($m) by Type, 2016
Figure 7.8 Zogenix: Revenue ($m) by Type, 2017
Figure 7.9 3M: Health Care Historical Revenue ($bn) and AGR (%), 2013-2017
Figure 7.10 BD: Historical Revenue ($bn) by Geographic Region, 2013-2017
Figure 7.11 BD Medical: Historical Revenue ($bn) and AGR (%), 2013-2015
Figure 7.12 Bioject Medical Technologies: Revenue Share (%) by Product Line, 2010
Figure 7.13 Bioject Medical Technologies: Revenue ($m), AGR (%), 2010-2014
Figure 8.1 The Transport Process for a Vaccine
Actavis
Activa Brand Products
AcuShot
Adamis
Aktiv-Dry
Alkermes
Alza
Anesiva
Antares Pharma
Aradigm
Astellas Pharma
AstraZeneca
BD
BeadTek
Berlex Labs
Bespak
bioCSL
Bioject Medical Technologies
Biomedics
Boehringer Ingelheim
Bristol-Myers Squibb and Somerset Pharmaceuticals
Cambridge Consultants
CareFusion Corporation
CEA-LETI
Corium International
CosMed
Crossject
CureVac
D’Antonio Consultants International
Debiotech
Durect
Echo Therapeutics
Elcam Medical
Eli Lilly & Co
Endo International
Ethical Holdings/Schering
Eurojet Medical
Ferndale
Georgia Institute of Technology
Giant Eagle
GlaxoSmithKline
Glide Pharmaceutical Technologies
Hangzhou Hema Medical Equipment
Haselmeir GmbH
Health-Mor Personal Care
Hirtenberger
Ichor
iHealthNet
Immunomic Therapeutics
ImmusanT
Injex Pharma
Inovio Pharmaceuticals
Invion
Janssen Pharmaceuticals
Key Pharmaceuticals
Kroger
LEO Pharma
LTS Lohmann Therapie-Systeme
MannKind
Mecaplast
Meda Pharmaceuticals
Medac Pharma
Medical International Technologies
Medi-Ject
MedImmune
Merck
Merck Serono
Micron Biomedical
Mylan
nanoBioSciences
NanoPass
Napp Pharmaceuticals
National Medical Products
Nektar Therapeutics
Nemaura
Nicobrand Limited
Novartis
Novo Nordisk
Novogyne Pharmaceuticals
NuPathe
Nycomed
Ortho-McNeil
Owen MumFord Inc
Par Pharmaceutical
Parke-Davis
Penjet Corporation
Permatec
Pernix
Pfizer
PharmaJet
PKA SoftTouch Corp
Procter & Gamble
ProStrakan
Purdue Pharma
Radius Health
Rhenovia Pharma
Rite Aid
Roche
Sandoz
Sanofi
Searle Pharmaceuticals
Shire
SHL Group AB
Shwarz-Pharma
Société Nationale des Poudres et Explosifs
STADA Arzneimittel AG
Stat Medical Devices
Takeda
Teva
Tev-Tropin
The Medical House
TheraJect
TheraTech
Transdermal Specialties
Trimeris
Trinity-Chiesi
UCB Pharma
University of California-San Francisco
Vaxxas
Vetter Pharma
Vyteris
Watson
West Pharmaceuticals
Westmount Pharmacy
Ypsomed AG
Zogenix
Zosano
Zydus Cadila
Organizations Mentioned in the report
Ankara University
Atlanta’s Emory University
Bill & Melinda Gates Foundation
Gwangju Institute of Science and Technology
Indian Institute of Science, Bangalore
Massachusetts Institute of Technology
National University of Singapore
Oxford University
Seoul National University
Shibaura Institute of Technology
University of Texas
University of Wisconsin’s Hospital
Download sample pages
Complete the form below to download your free sample pages for Needle-Free Delivery Technology Market 2018-2028
Related reports
-

Medical Device Leader Series: Top Pre-Filled Injection Device Manufacturers 2018-2028
The Pre-Filled Device Manufacturing market is estimated to reach $7.5bn in 2022, growing at a CAGR of 10.5% from 2017...Full DetailsPublished: 13 November 2018 -

Global Medical Device Contract Manufacturing Market Forecast 2018-2028
The global medical device contract manufacturing market was valued at $70bn in 2017. Visiongain forecasts this market to increase to...
Full DetailsPublished: 17 July 2018 -

Pharma Leader Series: Top 55 Pharmaceutical Contract Manufacturing Organisations (CMOs) Market 2020
Contract manufacturing represents the largest sector of the pharma outsourcing industry. Pharmaceutical companies have sought to take advantage of the...
Full DetailsPublished: 16 January 2020 -

Global Infusion Devices Market Forecast 2018-2028
The global infusion devices market was valued at $2.1bn in 2017 and is estimated to reach $3.9bn by 2028, growing...Full DetailsPublished: 16 August 2018 -

Generic Drugs Market Forecast 2019-2029
The generic drugs market is estimated to have reached $257.3bn in 2018 and is expected to grow at a CAGR...
Full DetailsPublished: 14 June 2019 -

Global Pre-Filled Syringes Market Forecast 2019-2029
The global pre-filled syringes market was valued at $9.8bn in 2018. This market is estimated to grow at a CAGR...
Full DetailsPublished: 31 January 2019 -

Global Pre-Filled Syringes Market Forecast 2017-2027
The global pre-filled syringes market is expected to grow at a moderate growth rate over the forecast period. The global...Full DetailsPublished: 31 March 2017 -

The Global Respiratory Inhalers Market 2018-2028
The global respiratory inhalers market reached $33bn in 2017 and is estimated to reach $38bn by 2023. In 2017, the...
Full DetailsPublished: 24 October 2018 -

Medical Devices Leader Series: Top In Vitro Diagnostics (IVD) Companies 2017-2027
What does the future hold for companies of the in vitro diagnostics (IVD) industry? Visiongain's new report Medical Devices Leader...Full DetailsPublished: 18 November 2016 -

Global Urology Devices Market Forecast 2019-2029
In 2018, the urology devices market is estimated at $6.9bn and is expected to grow at a CAGR of 6.1%...
Full DetailsPublished: 21 May 2019
Download sample pages
Complete the form below to download your free sample pages for Needle-Free Delivery Technology Market 2018-2028
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Don’t Miss Out!
Subscribe to receive the latest Industry news, trending insight and analysis straight to your inbox.Choose your preferences:
Latest Pharma news
Retinal Gene Therapy Market
The global Retinal Gene Therapy market is projected to grow at a CAGR of 9.6% by 2034
26 July 2024
HIV Drugs and Injectables Market
The global HIV Drugs & Injectables market is projected to grow at a CAGR of 4.6 % by 2034
24 July 2024
Digital Twin Technology in Pharmaceutical Manufacturing Market
The global Digital Twin Technology in Pharmaceutical Manufacturing market is projected to grow at a CAGR of 31.3% by 2034
23 July 2024
Specialty Pharma Market
The global Specialty Pharma market is projected to grow at a CAGR of 7.5% by 2034
22 July 2024