Industries > Pharma > Global Biosimilars and Follow-On Biologics Market 2020-2030
Global Biosimilars and Follow-On Biologics Market 2020-2030
Monoclonal Antibodies (mAbs), Fusion Proteins, Insulin, Erythropoietin’s, Granulocyte Colony-Stimulating Factor (G-CSF), Interferons, Growth Hormones, Fertility Hormones, Blood Disorders, Growth Hormone Deficiency, Autoimmune Diseases, Cancer, Diabetes, Infectious Diseases PLUS leading Biosimilars and Follow-On Biologics companies analysis
The global biosimilars and follow-on biologics market is estimated to have reached $19.87bn in 2019 and expected to grow at a CAGR of 7.1% in the first half of the forecast period. The market is dominated by Biosimilar Monoclonal Antibodies, this submarket is estimated to hold 36.5% share of this market in 2020.
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in this sector.
In this brand new 420-page report you will receive 131 tables and 90 figures– all unavailable elsewhere.
The 420-page report provides clear detailed insight into the global biosimilars and follow-on biologics market. Discover the key drivers and challenges affecting the market.
By ordering and reading our brand-new report today you stay better informed and ready to act.
Report Scope
• Global Biosimilars and Follow-on Biologics Market forecasts from 2020-2030
• Along with revenue prediction for the overall world market for biosimilars, our investigation shows forecasts to 2029 for 8 individual therapeutic submarkets:
• Monoclonal antibodies (mAbs)
• Fusion proteins
• Insulin
• Erythropoietin (EPO)
• Granulocyte colony-stimulating factor (G-CSF)
• Interferons
• Growth hormones
• Fertility hormones
• This report also shows revenue to 2030 for 11 individual submarkets within the above segments:
• Rituximab, infliximab, trastuzumab, adalimumab and bevacizumab
• Human insulin, insulin analogues, insulin glargine and insulin lispro
• Interferon alfa and interferon beta
• Our analyses show individual revenue forecasts to 2030 for 17 national markets:
• US
• Canada
• Japan
• Germany
• France
• UK
• Italy
• Spain
• Sweden
• Norway
• China
• India
• South Korea
• Russia
• Brazil
• South Africa
• Australia
• Our study discusses the leading companies that are involved in the biosimilars and follow-on biologics industry:
• Amgen Inc
• Biocon Limited
• Celltrion Healthcare Co. Ltd
• Dong-A Socio Holdings Co. Ltd
• Dr.Reddy’s Labs (DRL)
• & Other Companies
• Our study provides a SWOT analysis of the biosimilars and follow-on biologics market.
• Our study discusses pressures, opportunities and other events affecting the biosimilars industry and market, including these influences:
• Strategies for developing biosimilars – needs, demand, challenges and opportunities
• Guidelines from regulators (FDA, EMA and others)
• Patent challenges and data exclusivity for biopharmaceuticals
• Needs and opportunities in developing biosimilar mAbs, including rising incidence of cancers and increasing demand for lower-cost biologicals
• Developments in technology and operations for biosimilar drug production.
Visiongain’s study is intended for anyone requiring commercial analyses for the biosimilars and follow-on biologics market. You find data, trends and predictions.
Buy our report today Global Biosimilars and Follow-On Biologics Market: Monoclonal Antibodies (mAbs), Fusion Proteins, Insulin, Erythropoietin’s, Granulocyte Colony-Stimulating Factor (G-CSF), Interferons, Growth Hormones, Fertility Hormones, Blood Disorders, Growth Hormone Deficiency, Autoimmune Diseases, Cancer, Diabetes, Infectious Diseases.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: sara.peerun@visiongain.com
1 Report Overview
1.1 Biosimilar Overview
1.1.1 Global Biosimilars Market Forecast, 2020-2030
1.2 Biosimilars Market Segmentation
1.3 Why You Should Read this Report
1.4 Main Questions Answered by this Report
1.5 Who is this Report for?
1.6 Research and Analysis Methods
1.7 Frequently Asked Questions (FAQ)
1.8 Some Associated Reports
1.9 About Visiongain
2 An Introduction to Biosimilars and Biosimilar Drug Development
2.1 What are Biologics?
2.1.1 Biologics Can Be Very Effective but Also Very Expensive
2.1.2 Brief History of Biological Drug Development
2.1.3 Why are Biologics the Most Lucrative Products in The Global Pharmaceutical Market?
2.2 What are Biosimilars?
2.3 Brief History of Biosimilars
2.4 What are Interchangeable Biological Products and how do They Differ from Biosimilars?
2.4.1 FDA Guidelines on Prescription of Interchangeables Vs Biosimilars
2.4.2 Where Does the EMA Stand with Regard to Interchangeables?
2.4.3 Where do the EU5 and other European Countries Stand with Regard to Automatic Substitution?
2.4.4 Why Would Nations Oppose Automatic Substitution of Reference Biologics with Biosimilars?
2.5 Main Segments of the Overall Biosimilars Market
2.6 Definitions
2.6.1 EMA Terminology Related to Biosimilars
2.6.2 FDA Terminology Related to Biosimilars
2.6.3 WHO Terminology Related to Biosimilars
2.6.4 Differences Between Common Drug Classifications
3 Market and Technology Background
3.1 Lifecycle of a Biosimilar Drug
3.2 Development of a Biosimilar
3.2.1 Cell Line Development and Selection
3.2.2 E. coli Cells
3.3 Mammalian Cells
3.4 Manufacturing of a Biosimilar
3.5 Preclinical Studies and Validation of a Biosimilar
3.6 Clinical Trials
3.7 Approval by Regulatory Agencies
3.8 Pharmacovigilance/Post-Approval Monitoring
4 Global Biosimilar Market by Type
4.1 Global Biosimilars Market Forecast by Submarket, 202 b0-2030
4.2 Recombinant Hormones
4.2.1 Human Growth Hormone: First Extracted in 1958
4.2.2 Biosimilar Growth Hormones Market Forecast, 2020-2030
4.3 Fertility Hormones
4.3.1 Two Products Lead the Branded Fertility Hormone Market
4.3.2 Long-Acting Follicle Stimulating Hormone (FSH)
4.3.3 Branded Fertility Hormones Market Outlook
4.3.4 Rising Infertility to Drive Demand to 2030
4.3.5 Biosimilar Fertility Hormones Market Forecast, 2020-2030
4.3.6 Human Growth Hormone (HGH)
4.3.7 Follicle-Stimulating Hormone (FSH)
4.4 Recombinant Growth Factors
4.4.1 Erythropoietin (EPO)
4.4.2 Biosimilar EPO Market Forecast, 2020-2030
4.4.3 Granulocyte-Colony Stimulating Factor (G-CSF)
4.4.4 Biosimilar EPO Market Forecast, 2020-2030
4.5 Insulin
4.5.1 Global Biosimilar Insulin Revenue Forecast, 2020-2030
4.5.2 Global Biosimilar Insulin Market Share, 2020-2030
4.5.3 Biosimilar Human Insulin Submarket Forecast, 2020-2030
4.5.4 Biosimilar Insulin Analogues Submarket, 2020-2030
4.5.5 Biosimilar Insulin Analogue Revenue Forecast, 2020-2030
4.6 Biosimilar Insulin Glargine
4.6.1 Biosimilar Insulin Glargine: Revenue Forecast, 2020-2030
4.7 Biosimilar Insulin Lispro
4.7.1 Biosimilar Insulin Lispro: Revenue Forecast, 2020-2030
4.8 Monoclonal Antibodies (mAbs)
4.8.1 Biosimilar Monoclonal Antibodies Market and Submarkets Forecast, 2020-2030
4.8.2 Global Biosimilar Monoclonal Antibodies Market Share, 2020-2030
4.8.3 Biosimilar Rituximab
4.8.4 Biosimilar Infliximab
4.8.5 Biosimilar Trastuzumab
4.8.6 Biosimilar Adalimumab
4.8.7 Biosimilar Bevacizumab
4.9 Fusion Proteins
4.10 Interferons
4.10.1 Biosimilar Interferon Market Forecast, 2020-2030
4.10.2 Global Biosimilar Interferons Market Share, 2020-2030
4.11 Biosimilar Interferon Alfa
4.11.1 Biosimilar Interferon Alfa – a Common Target in Developing Countries, leading to Fragmented Markets
4.11.2 Biosimilar Peginterferon Alfa
4.11.3 Biosimilar Interferon Alfa Submarket Forecast, 2020-2030
4.12 Biosimilar Interferon Beta
4.12.1 Biosimilars are Well-Established in Emerging Markets
4.12.2 Biosimilar Interferon Beta Pipeline
4.12.3 Long-Acting Interferon Beta
4.12.4 Biosimilar Interferon Beta Submarket Forecast, 2020-2030
4.13 Strategies to Develop Biobetters
4.13.1 PEGylation
4.13.2 Glycosylation
4.13.3 Fusion Proteins
4.14 Others
4.14.1 Low Molecular Weight Heparins
4.15 FDA Guidance to LMWH Biosimilars
4.15.1 EMA Guidance to LMWH Biosimilars
5 Biosimilars: Qualitative Analysis and Industry Trends
5.1 Analysis of Market Opportunities
5.2 Strengths of Biosimilars Market
5.2.1 Growing Aging Population
5.2.2 Rising Incidence of Diseases
5.2.3 Patent Expirations
5.2.4 Growing Pipeline
5.2.5 Drive to Reduce Costs
5.2.6 Government Incentives
5.2.7 Incentives in Europe
5.2.8 Incentives in Indian Market
5.2.9 Collaborations and Partnerships
5.2.10 Mergers and Acquisitions
5.3 Weaknesses of Biosimilars Market
5.3.1 Manufacturing Process of Biosimilars
5.3.2 Process Definition and Testing and Validation
5.3.3 Lack of Skilled Personnel
5.3.4 Regulatory Guidelines
5.3.5 Interchangeability
5.3.6 Nomenclature of Biosimilars
5.3.7 Patent Litigation
5.4 Threats in Biosimilars Market
5.4.1 Unpredictable Regulatory Landscape
5.4.2 Prescription by Medical Practitioners/Pharmacies
5.4.3 Competition
5.5 Opportunities in Biosimilars Market
5.5.1 Emerging Markets
5.5.2 Technological Innovation and Improved Process Knowledge
5.5.3 Pricing
5.6 Biosimilars Product Pipeline
6 Global Biosimilars and Follow-on Biologics Market Breakdown by Application
6.1 Market by Biosimilar Applications
6.1.1 Global Biosimilar Market by Application: Revenue Forecast, 2020-2030
6.1.2 Global Biosimilar Application Market Share, 2020-2030
6.2 Cancer Biosimilars
6.2.1 Market Overview
6.3 Blood Disorders
6.3.1 Market Overview
6.3.2 Market Revenue
6.4 Diabetes
6.4.1 Market Overview
6.4.2 Market Revenue
6.5 Growth Hormone Deficiency
6.5.1 Market Overview
6.5.2 Market Revenue
6.6 Infectious Diseases
6.6.1 Market Overview
6.6.2 Market Revenue
6.7 Autoimmune Diseases
6.7.1 Market Overview
6.7.2 Market Revenue
6.8 Other Diseases
6.8.1 Market Overview
6.8.2 Market Revenue
7 Global Biosimilars Market Outlook by Country, 2020-2030
7.1 National Submarket Forecasts 2020-2030
7.1.1 Global Biosimilar Market by Nation: Revenue Forecast, 2020-2030
7.2 Regional Market Overview
7.2.1 Global Biosimilar Market Share by Nation, 2020-2030
7.3 US Biosimilars Market Outlook 2020-2030
7.3.1 FDA Finalises Three New Guidelines on 351(k) Applications in April 2015
7.3.2 FDA Finally Releases Guidance on Biosimilar Naming
7.3.3 Individual States Can Pass their Own Biosimilar Substitution Laws
7.3.4 US Biosimilars Market Forecast 2020-2030
7.4 Canada Biosimilar Market Outlook, 2020
7.4.1 Canada Biosimilar Market Forecast, 2020-2030
7.5 The EU Biosimilar Market: History and Current Status
7.5.1 History of EMA Guidelines and Updates
7.5.2 Biosimilar Uptake in Europe Varies between Nations
7.5.3 European Biosimilars Market Forecast, 2020-2030
7.5.4 Inflectra and Remsima in Europe - Demonstrates a Path for Other Biosimilar mAbs
7.6 German Biosimilar Market Outlook, 2020
7.6.1 German Biosimilar Market Forecast, 2020-2030
7.7 French Biosimilars Market Outlook, 2020
7.7.1 Biosimilar Substitution Passed but Requires Decrees to Come into Effect, Expected Sometime This Year
7.7.2 French Biosimilar Market Forecast, 2020-2030
7.8 UK Biosimilar Market Outlook, 2020
7.8.1 UK Biosimilar Market Forecast, 2020-2030
7.9 Italian Biosimilars Market Outlook, 2020
7.9.1 Italian Biosimilars Market Forecast, 2020-2030
7.10 Spanish Biosimilars Market Outlook, 2020
7.10.1 Spanish Biosimilar Market Forecast, 2020-2030
7.11 Sweden Biosimilars Market Outlook, 2020
7.11.1 Sweden Biosimilar Market Forecast, 2020-2030
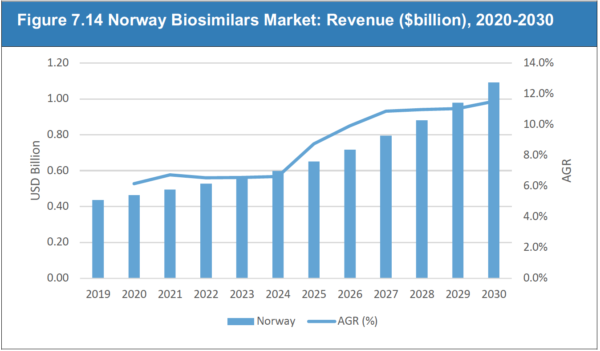
7.12 Norway Biosimilars Market Outlook, 2020
7.12.1 Norway Biosimilar Market Forecast, 2020-2030
7.13 Japan Biosimilars Market Outlook, 2020
7.13.1 Biosimilar Regulation in Japan and Currently Approved Biosimilars
7.13.2 Differences between the European and Japanese Guidelines
7.13.3 Currently Approved Biosimilars in Japan
7.13.4 Japanese Biosimilar Market Forecast, 2020-2030
8 Outlook for Biosimilars in Emerging Markets, 2020-2030
8.1 China and India Lead Biosimilar Revenues
8.2 Leading Emerging Biosimilar Markets Forecast, 2020-2030
8.3 Chinese Biosimilars Market Outlook, 2020
8.3.1 China Publishes Final Guidelines for Biosimilars
8.3.2 Chinese Biosimilars Market Forecast, 2020-2030
8.4 Indian Biosimilars Market Outlook, 2020
8.4.1 CDSCO Guidelines Released in 2012
8.4.2 Indian Biosimilars Market Forecast, 2020-2030
8.5 South Korea’s Established Biosimilar Guidelines
8.5.1 Currently Approved Biosimilars in South Korea, 2019
8.6 Russian Biosimilars Market Outlook and Forecast, 2020-2030
8.7 Brazilian Biosimilars Market Outlook and Forecast, 2020-2030
8.7.1 Brazilian Biosimilar Regulation
8.7.2 Government Eager to Promote Biosimilar Development, And Two Major Conglomerates, Bionovis And Orygen, Are Racing to Produce Biosimilars
8.7.3 Brazilian Biosimilars Market Forecast, 2020-2030
8.8 South Africa Biosimilars Market Outlook, 2020
8.8.1 South Africa Biosimilar Market Forecast, 2020-2030
8.9 Australia Biosimilars Market Outlook, 2020
8.9.1 Australia Biosimilar Market Forecast, 2020-2030
9 Industry Structure
9.1 Types of Market Players
9.1.1 Established Biologics Companies
9.1.2 Established Generics Companies
9.1.3 Bio-intellectual Companies
9.1.4 Opportunistic Companies
9.2 Emerging Trends in Biosimilar Industry Structure
9.2.1 Collaborations and Partnerships
9.2.2 Mergers and Acquisitions
9.2.3 Contract Manufacturing Organizations (CMOs)
9.3 Losses of Exclusivity- A Determining Factor
9.4 Biosimilar Market Entry Dates
9.5 Biosimilars in the Pipeline
9.5.1 Humira (adalimumab)
9.5.2 Enbrel (etanercept)
9.5.3 Lucentis (ranibizumab)
9.5.4 Actemra IV (tocilizumab)
9.5.5 Eylea (aflibercept)
9.6 Outsourcing Biosimilar Development
9.6.1 A Biosimilar Company Perspective
9.6.2 Business Model Differences
10 Leading Manufacturers/Suppliers of Biosimilar Drugs
10.1 Market Share
10.2 Recombinant Growth Factors
10.2.1 Market Share
10.3 Monoclonal Antibodies
10.3.1 Market Share
10.4 Fusion Proteins
10.4.1 Market Share
10.5 Others
10.5.1 Market Share
11 Company Profiles
11.1 Amgen Inc.
11.1.1 Company Overview
11.1.2 Company Financials
11.1.3 Company Recent Developments
11.2 Biocon Limited
11.2.1 Company Overview
11.2.2 Company Financials
11.2.3 Company Recent Developments
11.3 Celltrion Healthcare Co. Ltd.
11.3.1 Company Overview
11.3.2 Company Financials
11.3.3 Company Recent Developments
11.4 Dong-A Socio Holdings
11.4.1 Company Overview
11.4.2 Company Financials
11.4.3 Company Recent Developments
11.5 Dr. Reddy’s Laboratories Ltd.
11.5.1 Company Overview
11.5.2 Company Financials
11.5.3 Company Recent Developments
11.6 F Hoffmann-La Roche Ltd.
11.6.1 Company Overview
11.6.2 Company Financials
11.6.3 Company Recent Developments
11.7 Pfizer Inc.
11.7.1 Company Overview
11.7.2 Company Financials
11.7.3 Company Recent Developments
11.8 Stada Arzneimittel AG
11.8.1 Company Overview
11.8.2 Company Financials
11.8.3 Company Recent Developments
11.9 Teva Pharmaceutical Industries Ltd.
11.9.1 Company Overview
11.9.2 Company Financials
11.9.3 Company Recent Developments
11.10 Wockhardt Limited
11.10.1 Company Overview
11.10.2 Company Financials
11.10.3 Company Recent Developments
11.11 3S BIO INC.
11.11.1 Company Overview
11.11.2 Company Financials
11.12 CELLTRION INC.
11.12.1 Company Overview
11.12.2 Company Financials
11.13 GENEXINE INC.
11.13.1 Company Overview
11.14 MYLAN N.V.
11.14.1 Company Overview
11.14.2 Company Financials
11.15 ZYDUS CADILA
11.15.1 Company Overview
11.15.2 Company Financials
11.16 NIPPON KAYAKU CO. LTD.
11.16.1 Company Overview
11.16.2 Company Financials
11.17 MABXIENCE S.A.
11.17.1 Company Overview
11.18 LG LIFE SCIENCES
11.18.1 Company Overview
11.19 Intas Pharmaceuticals Ltd.
11.19.1 Company Overview
11.20 Gedeon Richter Plc
11.20.1 Company Overview
12 Global Biosimilar Patent Review
12.1 Patent Regulations in Favour of Branded Biologics
12.2 Market Exclusivity
12.2.1 Patent Dance
12.2.2 Licensing Notice
12.3 Patent Regulations in Favour of Biosimilars-Emerging Trends
12.3.1 Market Exclusivity Option
12.4 Patent Dance Developments
12.4.1 Licensing Notice
12.4.2 Product vs Process Patents
12.5 Patent Activity in Biosimilars, 2015-2019
12.5.1 United States Patents
12.5.2 Patents by Type
13 New Developments in Global Biosimilars Market
13.1 Major Companies in the Biosimilars Market
13.2 Biosimilars in Clinical Trials
13.3 Overview
13.3.1 Biosimilar Recombinant Hormones in Clinical Trials
13.4 Biosimilar Recombinant Growth Factors in Clinical Trials
13.5 Biosimilar Monoclonal Antibodies in Clinical Trials
13.6 Biosimilar Fusion Proteins in Clinical Trials
13.7 Other Biosimilars in Clinical Trials
13.8 Bio-betters in Clinical Trials
13.9 Significant Regulatory Activities
14 COVID-19 Impact on The Pharmaceutical Supply Chain
14.1 COVID-19 Impact on the Biosimilar Supply Chain
14.2 Health Care Services (HCPS) And Other Health Policy-Makers Unavailable:
14.3 Biosimilars See Some Positive Momentum, Despite Covid-19
14.4 Future Changes to Manufacturing Practices
15 Conclusions from Visiongain’s Research and Analysis
15.1 Rapid Growth Expected for this High-Potential Market
15.2 Biosimilar Monoclonal Antibodies to be the Fastest Growing Segment
15.3 A Range of Factors Will Stimulate Demand
15.4 Challenges Remain in Developing and Successfully Launching Biosimilars
Some Associated Reports
Visiongain Report Sales Order Form
Appendix A
About Visiongain
Appendix B
Visiongain report evaluation form
List of Tables
Table 1.1 Global Biosimilars Market Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 2.1 First Approvals for Recombinant Protein Therapies, 1982-1993
Table 2.2 Small Molecular Drugs vs. Biologics
Table 2.3 Generics vs. Biosimilars
Table 2.4 Biosimilars vs. Biobetters
Table 3.1 List of Biosimilars Produced in E. Coli
Table 3.2 List of Biosimilars Produced in Mammalian Cells
Table 3.3 Key Differences in Approval Pathways for Biosimilars, European Union (EU) vs. United Sates (U.S.)
Table 3.4 US Approved Biosimilars As of December 2019
Table 4.1 Biosimilars Market Forecast by Product Type: Revenue ($billion), AGR (%), CAGR (%),2020-2030
Table 4.2 Biosimilar Growth Hormone Market Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.3 Biosimilar Fertility Hormone Market Forecast: Revenue ($bn), AGR (%), CAGR (%), 2020-2030
Table 4.4 Biosimilar Recombinant Hormones
Table 4.5 Biosimilar EPO Market Forecast: Revenue ($billion), AGR (%), CAGR (%),2020-2030
Table 4.6 Biosimilar Recombinant Growth Factors
Table 4.7 Biosimilar G-CSF Market Forecast: Revenue ($billion), AGR (%), CAGR (%),2020-2030
Table 4.8 Biosimilar Insulin Market Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.9 Biosimilar Human Insulin Submarkets Forecast: Revenue ($billion), AGR (%), CAGR (%), 2019-2030
Table 4.10 Biosimilar Insulin Analogues Market Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.11 Biosimilar Insulin Glargine Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.12 Biosimilar Insulin Lispro Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.13 Biosimilar Monoclonal Antibodies
Table 4.14 Biosimilar Monoclonal Antibodies Market Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.15 Biosimilar Monoclonal Antibodies Market and Submarkets Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.16 Biosimilar Rituximab Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.17 Biosimilar Infliximab Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.18 Biosimilar Trastuzumab Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.19 Biosimilar Adalimumab Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.20 Biosimilar Bevacizumab Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.21 Biosimilar Fusion Proteins
Table 4.22 Fusion Proteins Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.23 Biosimilar Interferons Market Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.24 Selected Interferon Alfa Biosimilars Approved Worldwide, 2019
Table 4.25 Biosimilar Interferon Alfa Market Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.26 Selected Interferon Beta Biosimilars Approved Worldwide
Table 4.27 Biosimilar Interferon Alfa Market Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 4.28 Biosimilar LMWHs
Table 5.1 Biosimilars Product Pipeline
Table 6.1 Global Biosimilar Application Revenue Forecast by Type: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 6.2 Cancer Biosimilar Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 6.3 Blood Disorder Biosimilar Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 6.4 Diabetes Biosimilar Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 6.5 GHD Biosimilar Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 6.6 Infectious Disease Biosimilar Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 6.7 Autoimmune Diseases Biosimilar Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 6.8 Other Diseases Biosimilar Application Revenue Forecast: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 7.1 Biosimilars Market Forecast by Nations: Revenue ($billion), AGR (%), CAGR (%),2020-2030
Table 7.2 US Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%),2020-2030
Table 7.3 Canada Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%),2020-2030
Table 7.4: European Biosimilar Approvals To-Date, 2019
Table 7.5 European Biosimilar Market Segmented by Leading Nations: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 7.6 Germany Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 7.7 France Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%),2020-2030
Table 7.8 UK Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 7.9 Italy Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 7.10 Spain Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%),2020-2030
Table 7.11 Sweden Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%),2020-2030
Table 7.12 Norway Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%),2020-2030
Table 7.13: Japanese Biosimilar Approvals To-Date, 2019
Table 7.14 Japan Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%),2020-2030
Table 8.1 Leading Emerging Biosimilar Markets: Revenue ($billion), AGR (%), CAGR (%), Market Share (%), 2020-2030
Table 8.2 China Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 8.3 India Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 8.4: Currently Approved Biosimilars in South Korea, 2019
Table 8.5 South Korea Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 8.6 Russia Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 8.7 Comparison of Brazilian regulations for follow-on biologicals with EMA, FDA and WHO
Table 8.8 Brazil Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 8.9 South Africa Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%),2020-2030
Table 8.10 Australia Biosimilars Market: Revenue ($billion), AGR (%), CAGR (%), 2020-2030
Table 9.1 Mergers and Acquisitions in the Biosimilars Market, 2015-2020
Table 10.1 Leading Manufacturers/Suppliers of Recombinant Hormone Biosimilars, 2019
Table 10.2 Global Market Shares of Leading Manufacturers/Suppliers of Recombinant Hormone Biosimilars, 2019 (%)
Table 10.3 Leading Manufacturers/Suppliers of Recombinant Growth Factor Biosimilars, 2019
Table 10.4 Global Market Shares of Leading Manufacturers/Suppliers of Recombinant Growth Factor Biosimilars, 2019 (%)
Table 10.5 Leading Manufacturers/Suppliers of Monoclonal Antibody Biosimilars, 2019
Table 10.6 Global Market Shares of Leading Manufacturers/Suppliers of Monoclonal Antibody Biosimilars, 2019 (%)
Table 10.7 Leading Manufacturers/Suppliers of Fusion Protein Biosimilars, 2019
Table 10.8 Global Market Shares of Leading Manufacturers/Suppliers of Fusion Protein Biosimilars, 2019 (%)
Table 10.9 Leading Manufacturers/Suppliers of Other Biosimilars, 2019
Table 10.10 Global Market Shares of Leading Manufacturers/Suppliers of Other Biosimilars, 2019 (%)
Table 11.1 Amgen Inc. Profile 2019
Table 11.2 Company Financials, 2013-2018, USD Million
Table 11.3 Amgen: Key Developments
Table 11.4 Biocon Limited Profile 2020
Table 11.5 Biocon Limited Profile 2020
Table 11.6 Biocon Limited: Key Developments
Table 11.7 Celltrion Healthcare Co. Ltd. Profile 2019
Table 11.8 Company Financials, 2014-2018, USD Million
Table 11.9 Celltrion Healthcare: Key Developments
Table 11.10 Dong-A Socio Holdings Profile 2019
Table 11.11 Company Financials, 2014-2018, USD Million
Table 11.12 Dong-ASocio Holdings: Key Developments
Table 11.13 Dr. Reddy’s Laboratories Ltd. Profile 2020
Table 11.14 Company Financials, 2015-2019, USD Million
Table 11.15 Dr. Reddy’s Laboratories: Key Developments
Table 11.16 F Hoffmann-La Roche Ltd. Profile 2019
Table 11.17 Company Financials, 2014-2018, USD Million
Table 11.18 Roche Holding AG.: Key Developments
Table 11.19 Pfizer Inc. Profile 2019
Table 11.20 Company Financials, 2014-2018, USD Million
Table 11.21 Pfizer, Inc.: Key Developments
Table 11.22 Stada Arzneimittel AG Profile 2019
Table 11.23 Company Financials
Table 11.24 Stada: Key Developments
Table 11.25 Teva Pharmaceutical Industries Ltd. Profile 2019
Table 11.26 Company Financials, 2014-2018, USD Million
Table 11.27 Teva: Key Developments
Table 11.28 Wockhardt Limited Profile 2019
Table 11.29 Company Financials, 2017-2018, USD Million
Table 11.30 Wockhardt Limited: Key Developments
Table 11.31 Company Financials, 2016-2018, USD Million
Table 11.32 Company Financials, 2014-2018, USD Million
Table 11.33 Company Financials, 2014-2018, USD Million
Table 11.34 Company Financials, 2016-2019, USD Million
Table 11.35 Company Financials, 2015-2019, USD Million
Table 12.1 Inter Partes Review (IPR) Challenges by Biosimilar Developers
Table 12.2 Patents for Biosimilars, by Year, 2015-November 2019 (Number)
Table 12.3 Patents for Biosimilars, by Type, 2015-November 2019 (Number)
Table 12.4 US Approved Biosimilars
Table 12.5 European Approvals of Biosimilars
Table 13.1 Major Companies with Biosimilars in Pipeline
Table 13.2 Major Biosimilar Candidates with Patent Expirations
Table 13.3 Biosimilars in Various Phases of Clinical Trials, by Type
Table 13.4 Biosimilar Recombinant Hormones in Clinical Trials
Table 13.5 Biosimilar Recombinant Growth Factors in Clinical Trials
Table 13.6 Biosimilar Monoclonal Antibodies in Clinical Trials
Table 13.7 Biosimilar Fusion Proteins in Clinical Trials
Table 13.8 Other Biosimilars in Clinical Trials
Table 13.9 Representative Bio-betters in Clinical Trials
Table 13.10 List of Approved Biosimilar Drugs, by Country
List of Figures
Figure 1.1 Global Biosimilars Market Forecast: Revenue ($billion), 2020-2030
Figure 1.2 Main Segments and Sub-Segments of the Biosimilars Market, 2020
Figure 4.1 Global Biosimilars Market Forecasts by Submarket: Revenue ($billion), 2020-2030
Figure 4.2 Global Biosimilars Market Forecasts by Submarket: Revenue ($billion), 2020-2030
Figure 4.3 Biosimilar Fertility Hormone Market Forecast: Revenue ($bn), 2020-2030
Figure 4.4 Biosimilar EPO Market Forecast: Revenue ($billion), 2020-2030
Figure 4.5 Biosimilar G-CSF Market Forecast: Revenue ($billion), 2020-2030
Figure 4.6 Biosimilar Insulin Market Forecast: Revenue ($billion), 2020-2030
Figure 4.7 Biosimilar Insulin Market Share Forecast 2020
Figure 4.8 Biosimilar Insulin Market Share Forecast 2025
Figure 4.9 Biosimilar Insulin Market Share Forecast 2030
Figure 4.10 Biosimilar Human Insulin Submarket Revenue Forecast: Revenue ($billion), 2020-2030
Figure 4.11 Biosimilar Insulin Analogues Market Forecast: Revenue ($billion), 2020-2030
Figure 4.12 Biosimilar Insulin Glargine Revenue Forecast: Revenue ($billion), 2020-2030
Figure 4.13 Biosimilar Insulin Submarket Forecast: Revenue ($billion), 2020-2030
Figure 4.14 Biosimilar Monoclonal Antibodies Market Forecast: Revenue ($billion), 2020-2030
Figure 4.15 Biosimilar Monoclonal Antibody Market Forecast, Split by Product: Revenue ($billion), 2020-2030
Figure 4.16 Biosimilar Monoclonal Antibodies Market Share Forecast 2020
Figure 4.17 Biosimilar Monoclonal Antibodies Market Share Forecast 2025
Figure 4.18 Biosimilar Monoclonal Antibodies Market Share Forecast 2030
Figure 4.19 Biosimilar Rituximab Revenue Forecast: Revenue ($billion), 2020-2030
Figure 4.20 Biosimilar Infliximab Revenue Forecast: Revenue ($billion), 2020-2030
Figure 4.21 Biosimilar Trastuzumab Revenue Forecast: Revenue ($billion), 2020-2030
Figure 4.22 Biosimilar Adalimumab Revenue Forecast: Revenue ($billion), 2020-2030
Figure 4.23 Biosimilar Bevacizumab Revenue Forecast: Revenue ($billion), 2020-2030
Figure 4.24 Fusion Proteins Revenue Forecast: Revenue ($billion), 2020-2030
Figure 4.25 Biosimilar Interferons Market Forecast: Revenue ($billion), 2020-2030
Figure 4.26 Biosimilar Interferons Market Share Forecast 2020
Figure 4.27 Biosimilar Interferons Market Share Forecast 2025
Figure 4.28 Biosimilar Interferons Market Share Forecast 2030
Figure 4.29 Biosimilar Interferon Alfa Market Forecast: Revenue ($billion), 2020-2030
Figure 4.30 Biosimilar Interferon Alfa Market Forecast: Revenue ($billion), 2020-2030
Figure 5.1 SWOT Analysis of Biosimilar Market
Figure 6.1 Global Biosimilar Application Revenue Forecast by Type: Revenue ($billion), 2020-2030
Figure 6.2 Global Biosimilar Application Market Share Forecast 2020
Figure 6.3 Global Biosimilar Application Market Share Forecast 2025
Figure 6.4 Global Biosimilar Application Market Share Forecast 2030
Figure 6.5 Cancer Biosimilar Revenue Forecast: Revenue ($billion), 2020-2030
Figure 6.6 Blood Disorder Biosimilar Revenue Forecast: Revenue ($billion), 2020-2030
Figure 6.7 Diabetes Biosimilar Revenue Forecast: Revenue ($billion), 2020-2030
Figure 6.8 GHD Biosimilar Revenue Forecast: Revenue ($billion), 2020-2030
Figure 6.9 Infectious Disease Biosimilar Revenue Forecast: Revenue ($billion), 2020-2030
Figure 6.10 Autoimmune Diseases Biosimilar Revenue Forecast: Revenue ($billion), 2020-2030
Figure 6.11 Other Diseases Biosimilar Application Revenue Forecast: Revenue ($billion), 2020-2030
Figure 7.1 Global Biosimilars Market Forecasts by Nations: Revenue ($billion), 2020-2030
Figure 7.2 Global Biosimilar Market Share by Nation Forecast 2020
Figure 7.3 Global Biosimilar Market Share by Nation Forecast 2025
Figure 7.4 Global Biosimilar Market Share by Nation Forecast 2030
Figure 7.5 US Biosimilars Market: Revenue ($billion), 2020-2030, AGR (%)
Figure 7.6 Canada Biosimilars Market: Revenue ($billion), 2020-2030
Figure 7.7 European Biosimilars Market Segmented by Leading Nations: Revenue ($billion), 2020-2030
Figure 7.8 Germany Biosimilars Market: Revenue ($bn), 2019-2030
Figure 7.9 France Biosimilars Market: Revenue ($billion), 2020-2030
Figure 7.10 UK Biosimilars Market: Revenue ($billion), 2020-2030
Figure 7.11 Italy Biosimilars Market: Revenue ($billion), 2020-2030
Figure 7.12 Spain Biosimilars Market: Revenue ($billion), 2020-2030
Figure 7.13 Sweden Biosimilars Market: Revenue ($billion), 2020-2030
Figure 7.14 Norway Biosimilars Market: Revenue ($billion), 2020-2030
Figure 7.15 Japan Biosimilars Market: Revenue ($bn), 2020-2030
Figure 8.1 Leading Emerging Nations Biosimilar Market Shares: Revenues ($billion), 2019-2030
Figure 8.2 China Biosimilars Market: Revenue ($billion), 2020-2030
Figure 8.3 India Biosimilars Market: Revenue ($billion), 2020-2030
Figure 8.4 South Korea Biosimilars Market: Revenue ($billion), 2020-2030
Figure 8.5 Russia Biosimilars Market: Revenue ($billion), 2020-2030
Figure 8.6 Brazil Biosimilars Market: Revenue ($billion), 2020-2030
Figure 8.7 South Africa Biosimilars Market: Revenue ($billion), 2020-2030
Figure 8.8 Australia Biosimilars Market: Revenue ($billion), 2020-2030
Figure 9.1 Developed Markets Impact of Brand Losses of Exclusivity, 2013-2022, USD Bn
Figure 9.2 US Impact of Brand Losses of Exclusivity, 2013-2022, USD Bn
Figure 10.1 Global Market Shares of Leading Manufacturers/Suppliers of Recombinant Hormone Biosimilars, 2019
Figure 10.2 Global Market Shares of Leading Manufacturers/Suppliers of Recombinant Growth Factor Biosimilars, 2019
Figure 10.3 Global Market Shares of Leading Manufacturers/Suppliers of Monoclonal Antibody Biosimilars, 2019 (%)
Figure 10.4 Global Market Shares of Leading Manufacturers/Suppliers of Fusion Protein Biosimilars, 2019 (%)
Figure 10.5 Global Market Shares of Leading Manufacturers/Suppliers of Other Biosimilars, 2019 (%)
Figure 11.1 Company Financials, 2013-2018, USD Million
Figure 11.2 Company Financials, 2015-2018, USD Million
Figure 11.3 Company Financials, 2014-2018, USD Million
Figure 11.4 Company Financials, 2014-2018, USD Million
Figure 11.5 Company Financials, 2015-2019, USD Million
Figure 11.6 Company Financials, 2014-2018, USD Million
Figure 11.7 Company Financials, 2014-2018, USD Million
Figure 11.8 Company Financials, 2014-2018, USD Million
Figure 11.9 Company Financials, 2014-2018, USD Million
Figure 11.10 Company Financials, 2017-2018, USD Million
Figure 11.11 Company Financials, 2016-2018, USD Million
Figure 11.12 Company Financials, 2014-2018, USD Million
Figure 11.13 Company Financials, 2014-2018, USD Million
Figure 11.14 Company Financials, 2016-2019, USD Million
Figure 11.15 Company Financials, 2015-2019, USD Million
Figure 12.1 Top 10 selling biologics patent cliff (2000–2020)
Amgen Inc.
Biocon Limited
Bio-Manguinhos
Bionovis
BioXpress Therapeutics
Boehringer Ingelheim
Bristol-Myers Squibb
Cell Therapeutics
Celltrion Healthcare Co. Ltd.
Dong-A Socio Holdings
Dr. Reddy’s Laboratories Ltd.
Emcure
EMS
Epirus Biopharmaceuticals
Farmasa
Fresenius
Fujifilm Kyowa Kirin Biologics
F Hoffmann-La Roche Ltd.
Gedeon Richter Plc
GENEXINE INC.
Hanwha Chemical
Harvest Moon
Hospira
Hypermarcas
iBio
IBSS Biomed
ImClone LLC
Instituto Vital Brazil
IPCA Labs
Isu Abxis
Janssen
Johnson & Johnson
Kyowa Hakko Kirin
Laboratorios Liomont
Intas Pharmaceuticals
LG Life Sciences
MABXIENCE S.A.
Merck Serono
Millhouse LLC
Mitsubishi Tanabe
Mochida Pharmaceutical
Mustafa Nevzat Pharmaceuticals
Natco Pharma
Nichi-Iko Pharmaceutical Co.
Mylan N.V.
Nippon Kayaku Co. Ltd.
Pfizer Inc.
QuantiaMD
Quintiles
Reliance Life Sciences
Roche
Samsung Bioepis
Sandoz
Sanofi
Schnell
Seattle Genetics
Shanghai CP Guojian
Shionogi
Sorrento
Spectrum Pharmaceuticals
Stada Arzneimittel AG
Teva Pharmaceutical Industries Ltd.
UCB
União Química
Viropro
Walvax Biotechnology
Wyeth
Zenotech
Zhejiang Huahai Pharmaceutical
Wockhardt Limited
Zydus Cadila group
List of Organizations Mentioned in the Report
Agence française de sécurité sanitaire des produits de santé (ANSM)
Agência Nacional de Vigilância Sanitária (ANVISA)
Chinese Centre for Drug Evaluation (CDE)
Cour des Comptes (France)
European Medicines Agency (EMA)
Food and Drug Administration (US FDA)
Fraunhofer Center for Molecular Biology
Health Canada
India Brand Equity Foundation
Korean Food and Drug Administration (KFDA)
Medicines and Healthcare Products Regulatory Agency (MHRA)
Ministry of Food and Drug Safety (South Korea)
Ministry of Health (Russia)
Ministry of Health, Labour and Welfare (MHLW)
National Institute for Health and Care Excellence (NICE)
National Institute for Health Research Horizon Scanning Centre
Norwegian Medical Agency
Russian Ministry of Health
Scientific Centre for Expertise of Medicinal Application Products (Russia)
Spanish Ministry of Health
State Food and Drug Administration (SFDA)
The Cancer Centre Bahamas
Washington Legal Foundation
World Health Organization (WHO)
Download sample pages
Complete the form below to download your free sample pages for Global Biosimilars and Follow-On Biologics Market 2020-2030
Related reports
-

Cancer Biologics Market Report 2021-2031
The global demand for cancer biologics is expected to expand significantly over the projected period, owing to the expanded acceptance...Full DetailsPublished: 04 February 2021 -

Global Biosimilar Monoclonal Antibodies Forecast 2020-2030
The global biosimilar monoclonal antibodies market is expected to grow at a CAGR of 9.6% in the second half of...
Full DetailsPublished: 28 February 2020 -

Cancer Biologics Therapies Market Report 2021-2031
The main drivers for the growth include technological advancements in the development of novel biomarkers, rising preference for minimally or...Full DetailsPublished: 01 April 2021 -

Global Bioreactors Market 2020-2030
The global bioreactors market is expected to grow at a CAGR of 7.5% in the second half of the forecast...
Full DetailsPublished: 10 January 2020 -

Cancer Supportive Care Drugs Market Report 2020-2030
Cancer has been primarily leading causes of death worldwide and most treatments for cancer come with various side effects such...
Full DetailsPublished: 01 January 1970 -

Global Rheumatoid Arthritis Drugs Market Forecast 2020-2030
The global Rheumatoid Arthritis market is estimated to have reached $58bn in 2018 and is expected to grow at a...
Full DetailsPublished: 17 February 2020 -

Biologics Market Report 2020-2030
Over the years, the global biologics market has increased and estimates are made that in the next five years i.e....Full DetailsPublished: 04 August 2020 -

Biopharmaceuticals Contract Manufacturing Market Report 2020-2030
As of 2019, the global biopharmaceuticals market’s size was estimated at ~$232 billion. It is expected to grow at a...
Full DetailsPublished: 04 March 2020 -

Precision Cancer Diagnostic Test Market Report 2021-2031
Molecular diagnostics are rapidly transforming drug development and patient selection: trials with biomarkers have higher success rates than those without,...Full DetailsPublished: 12 November 2020 -

CRO Regulatory Services for Generics and Biosimilars Drugs Market Report 2020-2030
Growing demand for contract manufacturing services; concentrating on core competencies of pharmaceutical and biopharmaceutical companies; and increasing demand for biosimilars...Full DetailsPublished: 10 August 2020
Download sample pages
Complete the form below to download your free sample pages for Global Biosimilars and Follow-On Biologics Market 2020-2030
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Don’t Miss Out!
Subscribe to receive the latest Industry news, trending insight and analysis straight to your inbox.Choose your preferences:
Latest Pharma news
Retinal Gene Therapy Market
The global Retinal Gene Therapy market is projected to grow at a CAGR of 9.6% by 2034
26 July 2024
HIV Drugs and Injectables Market
The global HIV Drugs & Injectables market is projected to grow at a CAGR of 4.6 % by 2034
24 July 2024
Digital Twin Technology in Pharmaceutical Manufacturing Market
The global Digital Twin Technology in Pharmaceutical Manufacturing market is projected to grow at a CAGR of 31.3% by 2034
23 July 2024
Specialty Pharma Market
The global Specialty Pharma market is projected to grow at a CAGR of 7.5% by 2034
22 July 2024


















