The Decentralised Clinical Trials Market Report 2023-2033: This report will prove invaluable to leading firms striving for new revenue pockets if they wish to better understand the industry and its underlying dynamics. It will be useful for companies that would like to expand into different industries or to expand their existing operations in a new region.
The COVID-19 pandemic necessitated unprecedented adaptation on the part of life sciences organisations worldwide, for better or for worse, on a scale that has not been witnessed before in the contemporary industry. In response to the pandemic, biopharmaceutical companies and contract research organisations (CROs) have implemented decentralised or digital clinical trials (DCTs) as a means of minimising contact, enhancing the patient experience, and maintaining the progress of studies. The advantages of utilising virtual methods in the context of digital clinical trials are evident and have proven to be advantageous for life science entities. The clinical trial landscape has undergone a transformation with advancements in operational aspects such as patient engagement and site experience, cost reduction, and improved data integration. This shift has resulted in the acceptance of DCTs as a viable approach for conducting clinical studies.
Nevertheless, there are still obstacles to be overcome. The integration of conventional clinical trial obstacles with those arising from the shift towards digital solutions and decentralised designs characterises this novel clinical trial paradigm. The responsibility lies with biopharmaceutical companies and Contract Research Organizations (CROs) to comprehend and identify the challenges that arise during the implementation of a decentralised clinical trial. The achievement of academic success is contingent upon the assessment and integration of user-friendly remedies that can be expeditiously executed and simplified without imposing undue burden on healthcare professionals and recipients, all while upholding security and regulatory protocols.
What Questions Should You Ask before Buying a Market Research Report?
• How is the decentralised clinical trials market evolving?
• What is driving and restraining the decentralised clinical trials market?
• How will each decentralised clinical trials submarket segment grow over the forecast period and how much revenue will these submarkets account for in 2033?
• How will the market shares for each decentralised clinical trials submarket develop from 2023 to 2033?
• What will be the main driver for the overall market from 2023 to 2033?
• Will leading decentralised clinical trials markets broadly follow the macroeconomic dynamics, or will individual national markets outperform others?
• How will the market shares of the national markets change by 2033 and which geographical region will lead the market in 2033?
• Who are the leading players and what are their prospects over the forecast period?
• What are the decentralised clinical trials projects for these leading companies?
• How will the industry evolve during the period between 2023 and 2033? What are the implications of
decentralised clinical trials projects taking place now and over the next 10 years?
• Is there a greater need for product commercialisation to further scale the decentralised clinical trials market?
• Where is the decentralised clinical trials market heading and how can you ensure you are at the forefront of the market?
• What are the best investment options for new product and service lines?
• What are the key prospects for moving companies into a new growth path and C-suite?
You need to discover how this will impact the decentralised clinical trials market today, and over the next 10 years:
• Our 278-page report provides 120 tables and 158 charts/graphs exclusively to you.
• The report highlights key lucrative areas in the industry so you can target them – NOW.
• It contains in-depth analysis of global, regional and national sales and growth.
• It highlights for you the key successful trends, changes and revenue projections made by your competitors.
This report tells you TODAY how the decentralised clinical trials market will develop in the next 10 years, and in line with the variations in COVID-19 economic recession and bounce. This market is more critical now than at any point over the last 10 years.
Forecasts to 2033 and other analyses reveal commercial prospects
• In addition to revenue forecasting to 2033, our new study provides you with recent results, growth rates, and market shares.
• You will find original analyses, with business outlooks and developments.
• Discover qualitative analyses (including market dynamics, drivers, opportunities, and restraints), Porter’s Five Forces Analysis, PEST Analysis and recent developments.
This report includes data analysis and invaluable insight into how COVID-19 will affect the industry and your company. Four COVID-19 recovery patterns and their impact, namely, “V”, “L”, “W” and “U” are discussed in this report.
Segments Covered in the Report
Indication
• Oncology
• Cardiovascular
• Immunology
• Respiratory
• Others
Study Design
• Interventional
• Observational
• Expanded Access
End-users
• Pharmaceutical and Biopharmaceutical Companies
• CROs
• Others
Component
• Mobile Healthcare
• Telemedicine
• Wearable Devices
• Web-based Technology
• Others

In addition to the revenue predictions for the overall world market and segments, you will also find revenue forecasts for five regional and 23 leading national markets:
North America
• U.S.
• Canada
Europe
• Germany
• UK
• France
• Italy
• Spain
• Rest of Europe
Asia Pacific
• Japan
• China
• India
• Australia
• South Korea
• Singapore
• Rest of Asia Pacific
Latin America
• Brazil
• Mexico
• Argentina
• Colombia
• Rest of Latin America
MEA
• GCC
• South Africa
• Rest of MEA
Need industry data? Please contact us today.
The report also includes profiles and for some of the leading companies in the Decentralised Clinical Trials Market, 2023 to 2033, with a focus on this segment of these companies’ operations.
Leading companies and the potential for market growth
• CASTOR EDC
• Clinical ink
• CLOUDZBY, INC.
• Dassault Systèmes (Medidata Solutions, Inc.)
• ERGOMED PLC
• FLORENCE HEALTHCARE INC.
• ICON PLC
• IQVIA Holdings, Inc.
• Labcorp Drug Development
• Medable, Inc.
• Medrio Inc.
• Oracle Corporation
• Parexel International Corporation
• PPD (Thermo Fisher Scientific Inc.)
• Syneos Health
Overall world revenue for Decentralised Clinical Trials Market, 2023 to 2033 in terms of value the market will surpass US$8,125.0 million in 2023, our work calculates. We predict strong revenue growth through to 2033. Our work identifies which organizations hold the greatest potential. Discover their capabilities, progress, and commercial prospects, helping you stay ahead.
How will the Decentralised Clinical Trials Market, 2023 to 2033 report help you?
In summary, our 270+ page report provides you with the following knowledge:
• Revenue forecasts to 2033 for Decentralised Clinical Trials Market, 2023 to 2033 Market, with forecasts for indication, study design, end-users, and component, each forecast at a global and regional level – discover the industry’s prospects, finding the most lucrative places for investments and revenues.
• Revenue forecasts to 2033 for five regional and 23 key national markets – See forecasts for the Decentralised Clinical Trials Market, 2023 to 2033 market in North America, Europe, Asia-Pacific, Latin America, and MEA. Also forecasted is the market in the US, Canada, Mexico, Brazil, Germany, France, UK, Italy, China, India, Japan, and Australia among other prominent economies.
• Prospects for established firms and those seeking to enter the market – including company profiles for 15 of the major companies involved in the Decentralised Clinical Trials Market, 2023 to 2033.
Find quantitative and qualitative analyses with independent predictions. Receive information that only our report contains, staying informed with invaluable business intelligence.
Information found nowhere else
With our new report, you are less likely to fall behind in knowledge or miss out on opportunities. See how our work could benefit your research, analyses, and decisions. Visiongain’s study is for everybody needing commercial analyses for the Decentralised Clinical Trials Market, 2023 to 2033, market-leading companies. You will find data, trends and predictions.
To access the data contained in this document please email contactus@visiongain.com
Buy our report today Decentralised Clinical Trials Market Report 2023-2033: Forecasts by Indication (Oncology, Cardiovascular, Immunology, Respiratory, Others), by Study Design (Interventional, Observational, Expanded Access), by End-users (Pharmaceutical and Biopharmaceutical Companies, CROs, Others), by Component (Mobile Healthcare, Telemedicine, Wearable Devices, Web-based Technology, Others) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Impact and Recovery Pattern Analysis. Avoid missing out by staying informed – order our report now.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for a specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: contactus@visiongain.com
1 Report Overview
1.1 Objectives of the Study
1.2 Introduction to Decentralised Clinical Trials Market
1.3 What This Report Delivers
1.4 Why You Should Read This Report
1.5 Key Questions Answered by This Analytical Report Include:
1.6 Who is This Report For?
1.7 Methodology
1.7.1 Market Definitions
1.7.2 Market Evaluation & Forecasting Methodology
1.7.3 Data Validation
1.7.3.1 Primary Research
1.7.3.2 Secondary Research
1.8 Frequently Asked Questions (FAQs)
1.9 Associated Visiongain Reports
1.10 About Visiongain
2 Executive Summary
3 Market Overview
3.1 Key Findings
3.2 Market Dynamics
3.2.1 Market Driving Factors
3.2.1.1 Advantages of Decentralised Trials Driving Market Growth
3.2.1.2 Growing Adoption of Advanced Technologies is Boosting the Market Growth
3.2.1.3 COVID-19 Pandemic is driving the Adoption of Decentralised Clinical Trials
3.2.1.4 Rising Presence of CROs in Emerging Market is Boosting the Market Growth
3.2.2 Market Restraining Factors
3.2.2.1 Data Security Concerns Hinders the Market Growth
3.2.2.2 Complex Supply Chain and Manufacturing Could limit the Entry of New Players
3.2.3 Market Opportunities
3.2.3.1 Evolution of Decentralised Trials Technologies is Projected to Boost the Market Growth
3.2.3.2 Introduction of Advanced Cloud Platform
3.2.3.3 Support from Government is Estimated to Propel Market Growth
3.2.3.4 Mergers, Agreements, and Acquisitions by the Market Players is a Major Trend Witnessed in the Market
3.3 COVID-19 Impact Analysis
3.4 SWOT Analysis for Decentralised Clinical Trials Market
3.4.1 Strengths
3.4.2 Weaknesses
3.4.3 Opportunities
3.4.4 Threats
3.5 Porter’s Five Forces Analysis
3.5.1 Bargaining Power of Supplier
3.5.2 Bargaining Power of Buyer
3.5.3 Competitive Rivalry
3.5.4 Threat of New Entrants
3.5.5 Threat of Substitutes
3.6 PEST Analysis
3.6.1 Political Factors Impacting Decentralised Clinical Trials Market
3.6.2 Economic Factors Impacting Decentralised Clinical Trials Market
3.6.3 Social Factors Impacting Decentralised Clinical Trials Market
3.6.4 Technological Factors Impacting Decentralised Clinical Trials Market
4 Decentralised Clinical Trials Market Analysis by Indication
4.1 Key Findings
4.2 Indication Segment: Market Attractiveness Index
4.3 Decentralised Clinical Trials Market Size Estimation and Forecast by Indication
4.4 Oncology
4.4.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
4.4.2 Market Share by Region, 2023 & 2033 (%)
4.5 Cardiovascular
4.5.1 Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
4.5.2 Market Share by Region, 2023 & 2033 (%)
4.6 Immunology
4.6.1 Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
4.6.2 Market Share by Region, 2023 & 2033 (%)
4.7 Respiratory
4.7.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
4.7.2 Market Share by Region, 2023 & 2033 (%)
4.8 Others
4.8.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
4.8.2 Market Share by Region, 2023 & 2033 (%)
5 Decentralised Clinical Trials Market Analysis by Study Design
5.1 Key Findings
5.2 Study Design Segment: Market Attractiveness Index
5.3 Decentralised Clinical Trials Market Size Estimation and Forecast by Study Design
5.4 Interventional
5.4.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
5.4.2 Market Share by Region, 2023 & 2033 (%)
5.5 Observational
5.5.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
5.5.2 Market Share by Region, 2023 & 2033 (%)
5.6 Expanded Access
5.6.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
5.6.2 Market Share by Region, 2023 & 2033 (%)
6 Decentralised Clinical Trials Market Analysis by End-users
6.1 Key Findings
6.2 End-users Segment: Market Attractiveness Index
6.3 Decentralised Clinical Trials Market Size Estimation and Forecast by End-users
6.4 Pharmaceutical and Biopharmaceutical Companies
6.4.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
6.4.2 Market Share by Region, 2023 & 2033 (%)
6.5 CROs
6.5.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
6.5.2 Market Share by Region, 2023 & 2033 (%)
6.6 Others
6.6.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
6.6.2 Market Share by Region, 2023 & 2033 (%)
7 Decentralised Clinical Trials Market Analysis by Component
7.1 Key Findings
7.2 Component Segment: Market Attractiveness Index
7.3 Decentralised Clinical Trials Market Size Estimation and Forecast by Component
7.4 Mobile Healthcare
7.4.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
7.4.2 Market Share by Region, 2023 & 2033 (%)
7.5 Telemedicine
7.5.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
7.5.2 Market Share by Region, 2023 & 2033 (%)
7.6 Wearable Devices
7.6.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
7.6.2 Market Share by Region, 2023 & 2033 (%)
7.7 Web-based Technology
7.7.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
7.7.2 Market Share by Region, 2023 & 2033 (%)
7.8 Others
7.8.1 Market Forecast by Region, 2023-2033 (US$ Mn, AGR %)
7.8.2 Market Share by Region, 2023 & 2033 (%)
8 Decentralised Clinical Trials Market Analysis by Region
8.1 Key Findings
8.2 Regional Market Size Estimation and Forecast
9 North America Decentralised Clinical Trials Market Analysis
9.1 Key Findings
9.2 North America Decentralised Clinical Trials Market Attractiveness Index
9.3 Market Size by Country, 2023, 2028 & 2033 (US$ Mn)
9.4 Market Size Estimation and Forecast by Country, 2023-2033 (US$ Mn)
9.5 Market Size Estimation and Forecast by Indication, 2023-2033 (US$ Mn)
9.6 Market Size Estimation and Forecast by Study Design, 2023-2033 (US$ Mn)
9.7 Market Size Estimation and Forecast by End-users, 2023-2033 (US$ Mn)
9.8 Market Size Estimation and Forecast by Component, 2023-2033 (US$ Mn)
9.9 U.S. Decentralised Clinical Trials Market Analysis
9.9.1 Presence of Leading Players is a Major factor driving the U. S. Decentralised Clinical Trials Market
9.9.2 U. S. Decentralised Clinical Trials Market Growth is Primarily Attributable to Growing R&D Expenditure and Outsourcing Activities
9.10 Canada Decentralised Clinical Trials Market Analysis
9.10.1 Canada Market Anticipated to Witness the Fastest Growth in the North America Region
9.10.2 Growing Canadian research collaborations determined to Provide Infrastructure for Cell-Based Therapy Research
10 Europe Decentralised Clinical Trials Market Analysis
10.1 Key Findings
10.2 Europe Decentralised Clinical Trials Market Attractiveness Index
10.3 Market Size by Country, 2023, 2028 & 2033 (US$ Mn)
10.4 Market Size Estimation and Forecast by Country, 2023-2033 (US$ Mn)
10.5 Market Size Estimation and Forecast by Indication, 2023-2033 (US$ Mn)
10.6 Market Size Estimation and Forecast by Study Design, 2023-2033 (US$ Mn)
10.7 Market Size Estimation and Forecast by End-users, 2023-2033 (US$ Mn)
10.8 Market Size Estimation and Forecast by Component, 2023-2033 (US$ Mn)
10.9 Germany Decentralised Clinical Trials Market Analysis
10.10 UK Decentralised Clinical Trials Market Analysis
10.11 France Decentralised Clinical Trials Market Analysis
10.12 Italy Decentralised Clinical Trials Market Analysis
10.13 Spain Decentralised Clinical Trials Market Analysis
10.14 Russia Decentralised Clinical Trials Market Analysis
10.15 Rest of Europe Decentralised Clinical Trials Market Analysis
11 Asia Pacific Decentralised Clinical Trials Market Analysis
11.1 Key Findings
11.2 Asia Pacific Decentralised Clinical Trials Market Attractiveness Index
11.3 Market Size by Country, 2023, 2028 & 2033 (US$ Mn)
11.4 Market Size Estimation and Forecast by Country, 2023-2033 (US$ Mn)
11.5 Market Size Estimation and Forecast by Indication, 2023-2033 (US$ Mn)
11.6 Market Size Estimation and Forecast by Study Design, 2023-2033 (US$ Mn)
11.7 Market Size Estimation and Forecast by End-users, 2023-2033 (US$ Mn)
11.8 Market Size Estimation and Forecast by Component, 2023-2033 (US$ Mn)
11.9 Japan Decentralised Clinical Trials Market Analysis
11.10 China Decentralised Clinical Trials Market Analysis
11.11 India Decentralised Clinical Trials Market Analysis
11.12 Australia Decentralised Clinical Trials Market Analysis
11.13 South Korea Decentralised Clinical Trials Market Analysis
11.14 Singapore Decentralised Clinical Trials Market Analysis
11.15 Rest of Asia Pacific Decentralised Clinical Trials Market Analysis
12 Latin America Decentralised Clinical Trials Market Analysis
12.1 Key Findings
12.2 Latin America Decentralised Clinical Trials Market Attractiveness Index
12.3 Market Size by Country, 2023, 2028 & 2033 (US$ Mn)
12.4 Market Size Estimation and Forecast by Country, 2023-2033 (US$ Mn)
12.5 Market Size Estimation and Forecast by Indication, 2023-2033 (US$ Mn)
12.6 Market Size Estimation and Forecast by Study Design, 2023-2033 (US$ Mn)
12.7 Market Size Estimation and Forecast by End-users, 2023-2033 (US$ Mn)
12.8 Market Size Estimation and Forecast by Component, 2023-2033 (US$ Mn)
12.9 Brazil Decentralised Clinical Trials Market Analysis
12.10 Mexico Decentralised Clinical Trials Market Analysis
12.11 Argentina Decentralised Clinical Trials Market Analysis
12.12 Colombia Decentralised Clinical Trials Market Analysis
12.13 Rest of Latin America Decentralised Clinical Trials Market Analysis
13 MEA Decentralised Clinical Trials Market Analysis
13.1 Key Findings
13.2 MEA Decentralised Clinical Trials Market Attractiveness Index
13.3 Market Size by Country, 2023, 2028 & 2033 (US$ Mn)
13.4 Market Size Estimation and Forecast by Country, 2023-2033 (US$ Mn)
13.5 Market Size Estimation and Forecast by Indication, 2023-2033 (US$ Mn)
13.6 Market Size Estimation and Forecast by Study Design, 2023-2033 (US$ Mn)
13.7 Market Size Estimation and Forecast by End-users, 2023-2033 (US$ Mn)
13.8 Market Size Estimation and Forecast by Component, 2023-2033 (US$ Mn)
13.9 GCC Decentralised Clinical Trials Market Analysis
13.10 South Africa Decentralised Clinical Trials Market Analysis
13.11 Rest of MEA Decentralised Clinical Trials Market Analysis
14 Company Profiles
14.1 IQVIA Holdings, Inc.
14.1.1 Company Snapshot
14.1.2 Company Overview
14.1.3 Financial Analysis
14.1.3.1 Net Revenue, 2015-2022
14.1.3.2 Regional Market Shares, 2022
14.1.4 Product Benchmarking
14.1.5 Strategic Outlook
14.2 ICON PLC
14.2.1 Company Snapshot
14.2.2 Company Overview
14.2.3 Financial Analysis
14.2.3.1 Net Revenue, 2015-2022
14.2.3.2 Regional Market Shares, 2022
14.2.4 Product Benchmarking
14.2.5 Strategic Outlook
14.3 Labcorp Drug Development
14.3.1 Company Snapshot
14.3.2 Company Overview
14.3.3 Financial Analysis
14.3.3.1 Net Revenue, 2015-2022
14.3.4 Product Benchmarking
14.3.5 Strategic Outlook
14.4 Dassault Systèmes (Medidata Solutions, Inc.)
14.4.1 Company Snapshot
14.4.2 Company Overview
14.4.3 Financial Analysis
14.4.3.1 Net Revenue, 2015-2022
14.4.3.2 Regional Market Shares, 2022
14.4.4 Product Benchmarking
14.4.5 Strategic Outlook
14.5 Oracle Corporation
14.5.1 Company Snapshot
14.5.2 Company Overview
14.5.3 Financial Analysis
14.5.3.1 Net Revenue, 2015-2022
14.5.3.2 Regional Market Shares, 2022
14.5.4 Product Benchmarking
14.5.5 Strategic Outlook
14.6 Parexel International Corporation
14.6.1 Company Snapshot
14.6.2 Company Overview
14.6.3 Product Benchmarking
14.6.4 Strategic Outlook
14.7 Medrio Inc.
14.7.1 Company Snapshot
14.7.2 Company Overview
14.7.3 Product Benchmarking
14.7.4 Strategic Outlook
14.8 Medable, Inc.
14.8.1 Company Snapshot
14.8.2 Company Overview
14.8.3 Product Benchmarking
14.8.4 Strategic Outlook
14.9 Clinical ink
14.9.1 Company Snapshot
14.9.2 Company Overview
14.9.3 Product Benchmarking
14.9.4 Strategic Outlook
14.10 FLORENCE HEALTHCARE INC.
14.10.1 Company Snapshot
14.10.2 Company Overview
14.10.3 Product Benchmarking
14.10.4 Strategic Outlook
14.11 Syneos Health
14.11.1 Company Snapshot
14.11.2 Company Overview
14.11.3 Financial Analysis
14.11.3.1 Net Revenue, 2015-2022
14.11.3.2 Regional Market Shares, 2022
14.11.4 Product Benchmarking
14.11.5 Strategic Outlook
14.12 PPD (Thermo Fisher Scientific Inc.)
14.12.1 Company Snapshot
14.12.2 Company Overview
14.12.3 Financial Analysis
14.12.3.1 Net Revenue, 2016-2020
14.12.4 Product Benchmarking
14.12.5 Strategic Outlook
14.13 CASTOR EDC
14.13.1 Company Snapshot
14.13.2 Company Overview
14.13.3 Product Benchmarking
14.13.4 Strategic Outlook
14.14 CLOUDZBY, INC.
14.14.1 Company Snapshot
14.14.2 Company Overview
14.14.3 Product Benchmarking
14.14.4 Strategic Outlook
14.15 ERGOMED PLC
14.15.1 Company Snapshot
14.15.2 Company Overview
14.15.3 Financial Analysis
14.15.3.1 Net Revenue, 2016-2020
14.15.3.2 Regional Market Shares, 2022
14.15.4 Product Benchmarking
14.15.5 Strategic Outlook
15 Conclusion and Recommendations
15.1 Concluding Remarks from Visiongain
15.2 Recommendations for Market Players
List of Tables
Table 1 Decentralised Clinical Trials Market Snapshot, 2023 & 2033 (US$ million, CAGR %)
Table 2 Decentralised Clinical Trials Market Forecast by Region 2023-2033 (US$ Million, AGR%, CAGR%): "V" Shaped Recovery
Table 3 Decentralised Clinical Trials Market Forecast by Region 2023-2033 (US$ Million, AGR%, CAGR%): "U" Shaped Recovery
Table 4 Decentralised Clinical Trials Market Forecast by Region 2023-2033 (US$ Million, AGR%, CAGR%): "W" Shaped Recovery
Table 5 Decentralised Clinical Trials Market Forecast by Region 2023-2033 (US$ Million, AGR%, CAGR%): "L" Shaped Recovery
Table 6 Decentralised Clinical Trials Market Forecast by Indication, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 7 Oncology Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 8 Cardiovascular Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 9 Immunology Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 10 Respiratory Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 11 Others Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 12 Decentralised Clinical Trials Market Forecast by Study Design, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 13 Interventional Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 14 Observational Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 15 Expanded Access Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 16 Decentralised Clinical Trials Market Forecast by End-users, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 17 Pharmaceutical and Biopharmaceutical Companies Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 18 CROs Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 19 Others Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 20 Decentralised Clinical Trials Market Forecast by Component, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 21 Mobile Healthcare Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 22 Telemedicine Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 23 Wearable Devices Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 24 Web-based Technology Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 25 Others Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 26 Decentralised Clinical Trials Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 27 North America Decentralised Clinical Trials Market Forecast by Country, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 28 North America Decentralised Clinical Trials Market Forecast by Indication, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 29 North America Decentralised Clinical Trials Market Forecast by Study Design, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 30 North America Decentralised Clinical Trials Market Forecast by End-users, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 31 North America Decentralised Clinical Trials Market Forecast by Component, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 32 U.S. Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 33 Canada Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 34 Europe Decentralised Clinical Trials Market Forecast by Country, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 35 Europe Decentralised Clinical Trials Market Forecast by Indication, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 36 Europe Decentralised Clinical Trials Market Forecast by Study Design, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 37 Europe Decentralised Clinical Trials Market Forecast by End-users, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 38 Europe Decentralised Clinical Trials Market Forecast by Component, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 39 Germany Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 40 UK Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 41 France Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 42 Italy Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 43 Spain Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 44 Russia Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 45 Rest of Europe Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 46 Asia Pacific Decentralised Clinical Trials Market Forecast by Country, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 47 Asia Pacific Decentralised Clinical Trials Market Forecast by Indication, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 48 Asia Pacific Decentralised Clinical Trials Market Forecast by Study Design, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 49 Asia Pacific Decentralised Clinical Trials Market Forecast by End-users, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 50 Asia Pacific Decentralised Clinical Trials Market Forecast by Component, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 51 Japan Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 52 China Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 53 India Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 54 Australia Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 55 South Korea Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 56 Singapore Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 57 Rest of Asia Pacific Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 58 Latin America Decentralised Clinical Trials Market Forecast by Country, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 59 Latin America Decentralised Clinical Trials Market Forecast by Indication, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 60 Latin America Decentralised Clinical Trials Market Forecast by Study Design, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 61 Latin America Decentralised Clinical Trials Market Forecast by End-users, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 62 Latin America Decentralised Clinical Trials Market Forecast by Component, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 63 Brazil Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 64 Mexico Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 65 Argentina Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 66 Colombia Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 67 Rest of Latin America Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 68 MEA Decentralised Clinical Trials Market Forecast by Country, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 69 MEA Decentralised Clinical Trials Market Forecast by Indication, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 70 MEA Decentralised Clinical Trials Market Forecast by Study Design, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 71 MEA Decentralised Clinical Trials Market Forecast by End-users, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 72 MEA Decentralised Clinical Trials Market Forecast by Component, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 73 GCC Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 74 South Africa Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 75 Rest of MEA Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 76 IQVIA Holdings, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 77 IQVIA Holdings, Inc.: Product Benchmarking
Table 78 IQVIA Holdings, Inc.: Strategic Outlook
Table 79 ICON PLC: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 80 ICON PLC: Product Benchmarking
Table 81 ICON PLC: Strategic Outlook
Table 82 Labcorp Drug Development: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 83 Labcorp Drug Development: Product Benchmarking
Table 84 Labcorp Drug Development: Strategic Outlook
Table 85 Dassault Systèmes: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 86 Dassault Systèmes: Product Benchmarking
Table 87 Dassault Systèmes: Strategic Outlook
Table 88 Oracle Corporation: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 89 Oracle Corporation: Product Benchmarking
Table 90 Oracle Corporation: Strategic Outlook
Table 91 Paraxel International Corporation: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 92 Paraxel International Corporation: Product Benchmarking
Table 93 Paraxel International Corporation: Strategic Outlook
Table 94 Medrio Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 95 Medrio Inc.: Product Benchmarking
Table 96 Medrio Inc.: Strategic Outlook
Table 97 Medable, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 98 Medable Inc.: Product Benchmarking
Table 99 Medable Inc.: Strategic Outlook
Table 100 Clinical ink: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 101 Clinical ink: Product Benchmarking
Table 102 Clinical ink: Strategic Outlook
Table 103 Florence Healthcare, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 104 Florence Healthcare, Inc.: Product Benchmarking
Table 105 Florence Healthcare, Inc.: Strategic Outlook
Table 106 Syneos Health: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 107 Syneos Health: Product Benchmarking
Table 108 Syneos Health: Strategic Outlook
Table 109 PPD: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 110 PPD: Product Benchmarking
Table 111 PPD: Strategic Outlook
Table 112 CASTOR EDC: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 113 CASTOR EDC: Product Benchmarking
Table 114 CASTOR EDC: Strategic Outlook
Table 115 CLOUDZBY, INC.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 116 CLOUDZBY, INC.: Product Benchmarking
Table 117 CLOUDZBY, INC.: Strategic Outlook
Table 118 Ergomed PLC: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 119 Ergomed PLC: Product Benchmarking
Table 120 Ergomed PLC: Strategic Outlook
List of Figures
Figure 1 Decentralised Clinical Trials Market Segmentation
Figure 2 Decentralised Clinical Trials Market by Indication: Market Attractiveness Index
Figure 3 Decentralised Clinical Trials Market by Study Design: Market Attractiveness Index
Figure 4 Decentralised Clinical Trials Market by End-users: Market Attractiveness Index
Figure 5 Decentralised Clinical Trials Market by Component: Market Attractiveness Index
Figure 6 Decentralised Clinical Trials Market Attractiveness Index by Region
Figure 7 Decentralised Clinical Trials Market: Market Dynamics
Figure 8 Decentralised Clinical Trials Market by Region, 2023-2033 (US$ Mn, AGR %): “V” Shaped Recovery
Figure 9 Decentralised Clinical Trials Market by Region, 2023-2033 (US$ Mn, AGR %): “U” Shaped Recovery
Figure 10 Decentralised Clinical Trials Market by Region, 2023-2033 (US$ Mn, AGR %): “W” Shaped Recovery
Figure 11 Decentralised Clinical Trials Market by Region, 2023-2033 (US$ Mn, AGR %): “L” Shaped Recovery
Figure 12 Decentralised Clinical Trials Market: SWOT Analysis
Figure 13 Decentralised Clinical Trials Market: Porter’s Five Forces Analysis
Figure 14 Decentralised Clinical Trials Market Attractiveness Index by Indication
Figure 15 Decentralised Clinical Trials Market Forecast by Indication, 2023-2033 (US$ Million, AGR %)
Figure 16 Decentralised Clinical Trials Market Share Forecast by Indication, 2023, 2028, 2033 (%)
Figure 17 Oncology Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 18 Oncology Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 19 Cardiovascular Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 20 Cardiovascular Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 21 Immunology Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 22 Immunology Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 23 Respiratory Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 24 Respiratory Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 25 Others Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 26 Others Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 27 Decentralised Clinical Trials Market Attractiveness Index by Indication
Figure 28 Decentralised Clinical Trials Market Forecast by Study Design, 2023-2033 (US$ Million, AGR %)
Figure 29 Decentralised Clinical Trials Market Share Forecast by Study Design, 2023, 2028, 2033 (%)
Figure 30 Interventional Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 31 Interventional Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 32 Observational Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 33 Observational Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 34 Expanded Access Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 35 Expanded Access Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 36 Decentralised Clinical Trials Market Attractiveness Index by End-users
Figure 37 Decentralised Clinical Trials Market Forecast by End-users, 2023-2033 (US$ Million, AGR %)
Figure 38 Decentralised Clinical Trials Market Share Forecast by End-users, 2023, 2028, 2033 (%)
Figure 39 Pharmaceutical and Biopharmaceutical Companies Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 40 Pharmaceutical and Biopharmaceutical Companies Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 41 CROs Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 42 CROs Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 43 Others Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 44 Others Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 45 Decentralised Clinical Trials Market Attractiveness Index by Component
Figure 46 Decentralised Clinical Trials Market Forecast by Component, 2023-2033 (US$ Million, AGR %)
Figure 47 Decentralised Clinical Trials Market Share Forecast by Component, 2023, 2028, 2033 (%)
Figure 48 Mobile Healthcare Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 49 Mobile Healthcare Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 50 Telemedicine Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 51 Telemedicine Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 52 Wearable Devices Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 53 Wearable Devices Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 54 Web-based Technology Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 55 Web-based Technology Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 56 Others Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 57 Others Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 58 Decentralised Clinical Trials Market Forecast by Region 2023, 2028, 2033 (Revenue, CAGR%)
Figure 59 Decentralised Clinical Trials Market Share Forecast by Region 2023, 2028, 2033 (%)
Figure 60 Decentralised Clinical Trials Market by Region, 2023-2033 (US$ Mn, AGR %)
Figure 61 North America Decentralised Clinical Trials Market Attractiveness Index
Figure 62 North America Decentralised Clinical Trials Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 63 North America Decentralised Clinical Trials Market Forecast by Country, 2023-2033 (US$ million)
Figure 64 North America Decentralised Clinical Trials Market Share Forecast by Country, 2023 & 2033 (%)
Figure 65 North America Decentralised Clinical Trials Market Forecast by Indication, 2023-2033 (US$ Million, AGR %)
Figure 66 North America Decentralised Clinical Trials Market Share Forecast by Indication, 2023 & 2033 (%)
Figure 67 North America Decentralised Clinical Trials Market Forecast by Study Design, 2023-2033 (US$ Million, AGR %)
Figure 68 North America Decentralised Clinical Trials Market Share Forecast by Study Design, 2023 & 2033 (%)
Figure 69 North America Decentralised Clinical Trials Market Forecast by End-users, 2023-2033 (US$ Million, AGR %)
Figure 70 North America Decentralised Clinical Trials Market Share Forecast by End-users, 2023 & 2033 (%)
Figure 71 North America Decentralised Clinical Trials Market Forecast by Component, 2023-2033 (US$ Million, AGR %)
Figure 72 North America Decentralised Clinical Trials Market Share Forecast by Component, 2023 & 2033 (%)
Figure 73 U.S. Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 74 Canada Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 75 Europe Decentralised Clinical Trials Market Attractiveness Index
Figure 76 Europe Decentralised Clinical Trials Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 77 Europe Decentralised Clinical Trials Market Forecast by Country, 2023-2033 (US$ million)
Figure 78 Europe Decentralised Clinical Trials Market Share Forecast by Country, 2023 & 2033 (%)
Figure 79 Europe Decentralised Clinical Trials Market Forecast by Indication, 2023-2033 (US$ Million, AGR %)
Figure 80 Europe Decentralised Clinical Trials Market Share Forecast by Indication, 2023 & 2033 (%)
Figure 81 Europe Decentralised Clinical Trials Market Forecast by Study Design, 2023-2033 (US$ Million, AGR %)
Figure 82 Europe Decentralised Clinical Trials Market Share Forecast by Study Design, 2023 & 2033 (%)
Figure 83 Europe Decentralised Clinical Trials Market Forecast by End-users, 2023-2033 (US$ Million, AGR %)
Figure 84 Europe Decentralised Clinical Trials Market Share Forecast by End-users, 2023 & 2033 (%)
Figure 85 Europe Decentralised Clinical Trials Market Forecast by Component, 2023-2033 (US$ Million, AGR %)
Figure 86 Europe Decentralised Clinical Trials Market Share Forecast by Component, 2023 & 2033 (%)
Figure 87 Germany Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 88 UK Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 89 France Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 90 Italy Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 91 Spain Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 92 Russia Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 93 Rest of Europe Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 94 Asia Pacific Decentralised Clinical Trials Market Attractiveness Index
Figure 95 Asia Pacific Decentralised Clinical Trials Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 96 Asia Pacific Decentralised Clinical Trials Market Forecast by Country, 2023-2033 (US$ million)
Figure 97 Asia Pacific Decentralised Clinical Trials Market Share Forecast by Country, 2023 & 2033 (%)
Figure 98 Asia Pacific Decentralised Clinical Trials Market Forecast by Indication, 2023-2033 (US$ Million, AGR %)
Figure 99 Asia Pacific Decentralised Clinical Trials Market Share Forecast by Indication, 2023 & 2033 (%)
Figure 100 Asia Pacific Decentralised Clinical Trials Market Forecast by Study Design, 2023-2033 (US$ Million, AGR %)
Figure 101 Asia Pacific Decentralised Clinical Trials Market Share Forecast by Study Design, 2023 & 2033 (%)
Figure 102 Asia Pacific Decentralised Clinical Trials Market Forecast by End-users, 2023-2033 (US$ Million, AGR %)
Figure 103 Asia Pacific Decentralised Clinical Trials Market Share Forecast by End-users, 2023 & 2033 (%)
Figure 104 Asia Pacific Decentralised Clinical Trials Market Forecast by Component, 2023-2033 (US$ Million, AGR %)
Figure 105 Asia Pacific Decentralised Clinical Trials Market Share Forecast by Component, 2023 & 2033 (%)
Figure 106 Japan Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 107 China Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 108 India Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 109 Australia Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 110 South Korea Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 111 Singapore Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 112 Rest of Asia Pacific Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 113 Latin America Decentralised Clinical Trials Market Attractiveness Index
Figure 114 Latin America Decentralised Clinical Trials Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 115 Latin America Decentralised Clinical Trials Market Forecast by Country, 2023-2033 (US$ million)
Figure 116 Latin America Decentralised Clinical Trials Market Share Forecast by Country, 2023 & 2033 (%)
Figure 117 Latin America Decentralised Clinical Trials Market Forecast by Indication, 2023-2033 (US$ Million, AGR %)
Figure 118 Latin America Decentralised Clinical Trials Market Share Forecast by Indication, 2023 & 2033 (%)
Figure 119 Latin America Decentralised Clinical Trials Market Forecast by Study Design, 2023-2033 (US$ Million, AGR %)
Figure 120 Latin America Decentralised Clinical Trials Market Share Forecast by Study Design, 2023 & 2033 (%)
Figure 121 Latin America Decentralised Clinical Trials Market Forecast by End-users, 2023-2033 (US$ Million, AGR %)
Figure 122 Latin America Decentralised Clinical Trials Market Share Forecast by End-users, 2023 & 2033 (%)
Figure 123 Latin America Decentralised Clinical Trials Market Forecast by Component, 2023-2033 (US$ Million, AGR %)
Figure 124 Latin America Decentralised Clinical Trials Market Share Forecast by Component, 2023 & 2033 (%)
Figure 125 Brazil Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 126 Mexico Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 127 Argentina Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 128 Colombia Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 129 Rest of Latin America Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 130 MEA Decentralised Clinical Trials Market Attractiveness Index
Figure 131 MEA Decentralised Clinical Trials Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 132 MEA Decentralised Clinical Trials Market Forecast by Country, 2023-2033 (US$ million)
Figure 133 MEA Decentralised Clinical Trials Market Share Forecast by Country, 2023 & 2033 (%)
Figure 134 MEA Decentralised Clinical Trials Market Forecast by Indication, 2023-2033 (US$ Million, AGR %)
Figure 135 MEA Decentralised Clinical Trials Market Share Forecast by Indication, 2023 & 2033 (%)
Figure 136 MEA Decentralised Clinical Trials Market Forecast by Study Design, 2023-2033 (US$ Million, AGR %)
Figure 137 MEA Decentralised Clinical Trials Market Share Forecast by Study Design, 2023 & 2033 (%)
Figure 138 MEA Decentralised Clinical Trials Market Forecast by End-users, 2023-2033 (US$ Million, AGR %)
Figure 139 MEA Decentralised Clinical Trials Market Share Forecast by End-users, 2023 & 2033 (%)
Figure 140 MEA Decentralised Clinical Trials Market Forecast by Component, 2023-2033 (US$ Million, AGR %)
Figure 141 MEA Decentralised Clinical Trials Market Share Forecast by Component, 2023 & 2033 (%)
Figure 142 GCC Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 143 South Africa Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
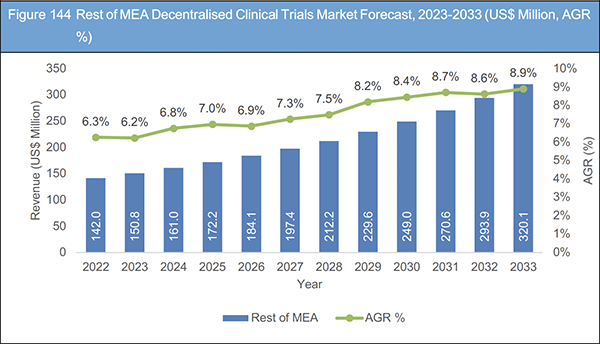
Figure 144 Rest of MEA Decentralised Clinical Trials Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 145 IQVIA Holdings Inc.: Net Revenue, 2015-2022 (US$ Million, AGR %)
Figure 146 IQVIA Holdings Inc.: Regional Market Shares, 2022
Figure 147 ICON PLC: Net Revenue, 2015-2022 (US$ Million, AGR %)
Figure 148 ICON PLC: Regional Market Shares, 2022
Figure 149 Labcorp Drug Development (Covance, Inc.): Net Revenue, 2015-2022 (US$ Million, AGR %)
Figure 150 Dassault Systèmes: Net Revenue, 2015-2022 (US$ Million, AGR %)
Figure 151 Dassault Systèmes: Regional Market Shares, 2022
Figure 152 Oracle Corporation: Net Revenue, 2015-2022 (US$ Million, AGR %)
Figure 153 Oracle Corporation: Regional Market Shares, 2022
Figure 154 Syneos Health: Net Revenue, 2015-2022 (US$ Million, AGR %)
Figure 155 Syneos Health: Regional Market Shares, 2022
Figure 156 PPD: Net Revenue, 2016-2020 (US$ Million, AGR %)
Figure 157 Ergomed PLC: Net Revenue, 2016-2020 (US$ Million, AGR %)
Figure 158 Ergomed PLC: Regional Market Shares, 2022
List of Companies Profiled in the Report
CASTOR EDC
Clinical ink
CLOUDZBY, INC.
Dassault Systèmes (Medidata Solutions, Inc.)
ERGOMED PLC
FLORENCE HEALTHCARE INC.
ICON PLC
IQVIA Holdings, Inc.
Labcorp Drug Development
Medable, Inc.
Medrio Inc.
Oracle Corporation
Parexel International Corporation
PPD (Thermo Fisher Scientific Inc.)
Syneos Health
List of Other Companies Mentioned in the Report
ADAMAS Consulting Group Limited
Aetion
AliveCor
Allucent
Aural Analytics
Biobeat
BioNTech
Blue Spark Technologies
Boco Digital Media, LLC
Boehringer Ingelheim
Circuit Clinical
Clalit Health Services
Click Therapeutics
ClinChoice
Clinical Research Malaysia
Cognizant
Compliance Quest
ConcertAI, LLC
Cryoport, Inc.
CVS Health
Datavant
Digital Artefacts
Equicare
Every Cure
Fosun Pharma USA Inc.
Genexine
GI Partners
GlobalCare
Glooko
GSK
Haystack Health
HealthCore
HealthVerity, Inc.
HMD Clinical
Illingworth Research Group
Indie Health
Janssen
LEO Pharma
Lightship
Lupus Therapeutics
Matrix Medical Network
Microsoft
Mural Health
Odaseva
Pfizer
PHARMASEAL International Ltd.
PHASTAR
PRA Health Sciences, Inc.
Q² Solutions
RxDataScience
Science 37
Seqster
Servier
StudyKIK
Tasso
Tech Mahindra
Trialbee
TriNetX
Vault Health, Inc.
Veeva System
Withings Health Solutions
List of Associations Mentioned in the Report
American Telemedicine Association (ATA)
Association for Clinical Data Management (ACDM)
Association of Clinical Research Organizations (ACRO)
Center for Biologics Evaluation and Research (CBER)
Center for Devices and Radiological Health (CDRH)
Center of Excellence in Immunology (CEI)
Centers for Disease Control and Prevention (CDC)
European Medicines Agency (EMA)
Food and Drug Administration (FDA)
Global Genes RARE Corporate Alliance
Indian Council of Medical Research (ICMR)
Institute for Healthcare Improvement (IHI)
International Agency for Research on Cancer (IARC)
Medicines and Healthcare Products Regulatory Agency (MHRA)
Ministry of Health, Labour & Welfare (MHLW)
National Cancer Institute (NCI)
National Cancer Institute's Center for Cancer Research (CCR)
National Center for Biotechnology Information (NCBI)
National Development and Reform Commission (NDRC)
National Institutes of Health (NIH)
United Nations (UN)
World Health Organization (WHO)





























