Industries > Pharma > Viral Vectors & Plasmid DNA Manufacturing Market Report 2022-2032
Viral Vectors & Plasmid DNA Manufacturing Market Report 2022-2032
Forecasts by Vector Type (Adenovirus, Retrovirus, Plasmid DNA, AAV, Lentivirus, Others), by Application (Antisense & RNAi, Gene Therapy, Cell Therapy, Vaccinology), by End-use (Pharma and Biopharma Companies, Research Institutes), by Disease (Oncology, Genetic Disorders, Infectious Diseases, Others), by Workflow (Upstream, Downstream) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Impact and Recovery Pattern Analysis
The Viral Vectors and Plasmid DNA Manufacturing Market Report 2022-2032: This report will prove invaluable to leading firms striving for new revenue pockets if they wish to better understand the industry and its underlying dynamics. It will be useful for companies that would like to expand into different industries or to expand their existing operations in a new region.
Viral-Vector Gene Therapy Offers Lucrative Opportunities
The long anticipated medicines are beginning to live up to their promise and have a significant impact on patient health thanks to the approval of a number of gene therapy products in recent years. Gene treatments are a novel medical strategy that have advanced as a result of extensive research, industry best practises, and challenging lessons. Gene therapy is still in its infancy, despite the fact that there are more and more medications in development and clinical trials every year. Manufacturers today face fresh issues and difficulties at every stage of the drug-commercialization process, from research and development to production and regulatory approval of AAV-based medicines. While this emerging industry can profit from the expertise amassed in the recombinant antibody space.
Costs and speed to market are important factors in process development. It is crucial to choose a production system that produces products more efficiently, with high yields, and with a high proportion of full to empty AAV. Transfection and adherent cells are currently being used by a number of businesses to reach the market since they offer a quick path there. The capacity to produce viral vectors is thought to be between one and two orders of magnitude less than what is required to meet both the present and future demands for commercial supply. As a result, the entire sector is concentrating on what is required to achieve sustained growth in capacity. The development of manufacturing procedures that can boost productivity is continuous, in addition to expanding manufacturing capacity through current and new manufacturing facilities. This entails improving plasmid constructions and cell lines, as well as process recovery in downstream processing.
What Questions Should You Ask before Buying a Market Research Report?
• How is the viral vectors and plasmid DNA manufacturing market evolving?
• What is driving and restraining the viral vectors and plasmid DNA manufacturing market?
• How will each viral vectors and plasmid DNA manufacturing submarket segment grow over the forecast period and how much revenue will these submarkets account for in 2032?
• How will the market shares for each viral vectors and plasmid DNA manufacturing submarket develop from 2022 to 2032?
• What will be the main driver for the overall market from 2022 to 2032?
• Will leading viral vectors and plasmid DNA manufacturing markets broadly follow the macroeconomic dynamics, or will individual national markets outperform others?
• How will the market shares of the national markets change by 2032 and which geographical region will lead the market in 2032?
• Who are the leading players and what are their prospects over the forecast period?
• What are the viral vectors and plasmid DNA manufacturing projects for these leading companies?
• How will the industry evolve during the period between 2022 and 2032? What are the implications of
viral vectors and plasmid DNA manufacturing projects taking place now and over the next 10 years?
• Is there a greater need for product commercialisation to further scale the viral vectors and plasmid DNA manufacturing market?
• Where is the viral vectors and plasmid DNA manufacturing market heading and how can you ensure you are at the forefront of the market?
• What are the best investment options for new product and service lines?
• What are the key prospects for moving companies into a new growth path and C-suite?
You need to discover how this will impact the viral vectors and plasmid DNA manufacturing market today, and over the next 10 years:
• Our 403-page report provides 152 tables and 177 charts/graphs exclusively to you.
• The report highlights key lucrative areas in the industry so you can target them – NOW.
• It contains in-depth analysis of global, regional and national sales and growth.
• It highlights for you the key successful trends, changes and revenue projections made by your competitors.
This report tells you TODAY how the viral vectors and plasmid DNA manufacturing market will develop in the next 10 years, and in line with the variations in COVID-19 economic recession and bounce. This market is more critical now than at any point over the last 10 years.
Forecasts to 2032 and other analyses reveal commercial prospects
• In addition to revenue forecasting to 2032, our new study provides you with recent results, growth rates, and market shares.
• You will find original analyses, with business outlooks and developments.
• Discover qualitative analyses (including market dynamics, drivers, opportunities, and restraints), Porter’s Five Forces Analysis, PEST Analysis and recent developments.
This report includes data analysis and invaluable insight into how COVID-19 will affect the industry and your company. Four COVID-19 recovery patterns and their impact, namely, “V”, “L”, “W” and “U” are discussed in this report.
Segments Covered in the Report
Vector Type
• Adenovirus
• Retrovirus
• Plasmid DNA
• AAV
• Lentivirus
• Others
Application
• Antisense & RNAi
• Gene Therapy
• Cell Therapy
• Vaccinology
End-use
• Pharma and Biopharma Companies
• Research Institutes
Disease
• Oncology
• Genetic Disorders
• Infectious Diseases
• Others
Workflow
• Upstream
• Downstream
In addition to the revenue predictions for the overall world market and segments, you will also find revenue forecasts for five regional and 21 leading national markets:
North America
• U.S.
• Canada
Europe
• Germany
• UK
• France
• Italy
• Spain
• Rest of Europe
Asia Pacific
• Japan
• China
• India
• Australia
• South Korea
• Rest of Asia Pacific
Latin America
• Brazil
• Mexico
• Argentina
• Rest of Latin America
MEA
• GCC
• South Africa
• Rest of MEA
Need industry data? Please contact us today.
The report also includes profiles and for some of the leading companies in the Viral Vectors and Plasmid DNA Manufacturing Market, 2022 to 2032, with a focus on this segment of these companies’ operations.
Leading companies and the potential for market growth
• Advanced BioScience Laboratories, Inc. (ABL, Inc.)
• Batavia Biosciences
• BioMarin
• BioNTech IMFS
• Biovian Oy
• bluebird bio, Inc.
• Creative Biogene
• CEVEC Pharmaceuticals GmbH
• Charles River Laboratories
• Cytiva
• FUJIFILM Diosynth Biotechnologies
• Genezen
• Lonza
• Merck KGaA
• Novasep (Thermofisher Scientific Inc.)
• Patheon Pharma Services (Thermo Fisher Scientific Inc.)
• Paragon Bioservices, Inc. (Catalent)
• Sirion-Biotech GmbH
• VGXI, Inc.
• Vibalogics
• Virovek
• Vivebiotech
• Waisman Biomanufacturing
• Wuxi AppTec, Yposkesi, Inc.
• Yposkesi, Inc.
Overall world revenue for Viral Vectors and Plasmid DNA Manufacturing Market, 2022 to 2032 in terms of value the market will surpass US$555 million in 2022, our work calculates. We predict strong revenue growth through to 2032. Our work identifies which organizations hold the greatest potential. Discover their capabilities, progress, and commercial prospects, helping you stay ahead.
How will the Viral Vectors and Plasmid DNA Manufacturing Market, 2022 to 2032 report help you?
In summary, our 400+ page report provides you with the following knowledge:
• Revenue forecasts to 2032 for Viral Vectors and Plasmid DNA Manufacturing Market, 2022 to 2032 Market, with forecasts for vector type, application, end-use, disease, and workflow, each forecast at a global and regional level – discover the industry’s prospects, finding the most lucrative places for investments and revenues.
• Revenue forecasts to 2032 for five regional and 21 key national markets – See forecasts for the Viral Vectors and Plasmid DNA Manufacturing Market, 2022 to 2032 market in North America, Europe, Asia-Pacific, Latin America, and MEA. Also forecasted is the market in the US, Canada, Mexico, Brazil, Germany, France, UK, Italy, China, India, Japan, and Australia among other prominent economies.
• Prospects for established firms and those seeking to enter the market – including company profiles for 25 of the major companies involved in the Viral Vectors and Plasmid DNA Manufacturing Market, 2022 to 2032.
Find quantitative and qualitative analyses with independent predictions. Receive information that only our report contains, staying informed with invaluable business intelligence.
Information found nowhere else
With our new report, you are less likely to fall behind in knowledge or miss out on opportunities. See how our work could benefit your research, analyses, and decisions. Visiongain’s study is for everybody needing commercial analyses for the Viral Vectors and Plasmid DNA Manufacturing Market, 2022 to 2032, market-leading companies. You will find data, trends and predictions.
To access the data contained in this document please email contactus@visiongain.com
Buy our report today Viral Vectors & Plasmid DNA Manufacturing Market Report 2022-2032: Forecasts by Vector Type (Adenovirus, Retrovirus, Plasmid DNA, AAV, Lentivirus, Others), by Application (Antisense & RNAi, Gene Therapy, Cell Therapy, Vaccinology), by End-use (Pharma and Biopharma Companies, Research Institutes), by Disease (Oncology, Genetic Disorders, Infectious Diseases, Others), by Workflow (Upstream, Downstream) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Impact and Recovery Pattern Analysis. Avoid missing out by staying informed – order our report now.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for a specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: contactus@visiongain.com
1.1 Objectives of the Study
1.2 Introduction to Viral Vectors and Plasmid DNA Manufacturing Market
1.3 What This Report Delivers
1.4 Why You Should Read This Report
1.5 Key Questions Answered By This Analytical Report Include:
1.6 Who is This Report For?
1.7 Methodology
1.7.1 Market Definitions
1.7.2 Market Evaluation & Forecasting Methodology
1.7.3 Data Validation
1.7.3.1 Primary Research
1.7.3.2 Secondary Research
1.8 Frequently Asked Questions (FAQs)
1.9 Associated Visiongain Reports
1.10 About Visiongain
2 Executive Summary
3 Market Overview
3.1 Key Findings
3.2 Market Dynamics
3.2.1 Market Driving Factors
3.2.1.1 Robust Pipeline for Gene Therapy and Viral Vectors
3.2.1.2 Increasing Capacities by Manufacturers
3.2.1.3 Advances in Genome Sequencing to Fuel Market Growth
3.2.2 Market Restraining Factors
3.2.2.1 High Cost of Therapies
3.2.2.2 Bottlenecks Experienced by Manufacturers
3.2.3 Market Opportunities
3.2.3.1 Rise in the Development of Allogenic and Autologous Cell Therapy
3.2.3.2 Increasing Applications As Preclinical Tool In Neuroscience Research
3.2.3.3 Increasing Number of Clinical Studies
3.3 COVID-19 Impact Analysis
3.4 Porter’s Five Forces Analysis
3.4.1 Supplier Power
3.4.2 Buyer Power
3.4.3 Competitive Rivalry
3.4.4 Threat from Substitutes
3.4.5 Threat of New Entrants
4 Viral Vector Production Capacity Mapping Analysis
4.1 CMOs Capacity for Viral Vector Manufacturing
4.2 Cleanroom Suites Facilities: Leading Viral Vector Manufacturers
5 Viral Vector Production & Yield Analysis
5.1 Introduction
5.2 ATMP Vectors and Manufacturing Platforms
5.3 Regulatory Challenges for Viral Vectors for Cell and Gene Therapy
6 Viral Vector Manufacturing: Process Economic Considerations and Challenges
6.1 Technological Advances in Manufacturing
6.2 Stable Producer Cell Lines
6.3 Addressing the Challenges of Cytotoxicity with Stable Producer Lines
6.4 What are the Process Development Considerations for the Use of Stable Producer Lines?
6.5 How Do Stable Producer Cell Lines Create Efficiencies and Reduce Costs in the Production of CGTs?
6.5.1 Simplified Upstream Production
6.5.2 Lower Costs for Reagents and Labour
6.5.3 More Flexibility for Scale Up
6.6 When is the Time to Switch from Transient Transfection to Stable Producer Cell Lines for LVV Manufacturing?
6.7 Cost Considerations
6.8 Regulatory Expectations
7 Viral Vectors Production Process Analysis
7.1 Introduction
7.2 Upstream Unit Operations (Cell Thaw and Expansion Through Transfection)
7.3 Downstream Unit Operations (Harvest Through Purification)
7.4 Formulation/Stability and Fill/Finish
7.5 Viral Analytics
7.6 Viral Vector Production: Facilities Needs
7.7 Regulatory Standard Needs
7.8 Workforce Development and Needs
8 Viral Vectors and Plasmid DNA Manufacturing Market Analysis by Vector Type
8.1 Key Findings
8.2 Vector Type Segment: Market Attractiveness Index
8.3 Viral Vectors and Plasmid DNA Manufacturing Market Size Estimation and Forecast by Vector Type
8.4 Adenovirus
8.4.1 Market Forecast by Region, 2022-2032 (US$ Mn)
8.4.2 Market Share by Region, 2022 & 2032 (%)
8.5 Retrovirus
8.5.1 Market Forecast by Region, 2022-2032 (US$ Mn)
8.5.2 Market Share by Region, 2022 & 2032 (%)
8.6 Plasmid DNA
8.6.1 Market Forecast by Region, 2022-2032 (US$ Mn)
8.6.2 Market Share by Region, 2022 & 2032 (%)
8.7 AAV (Adeno-associated Viruses)
8.7.1 Market Forecast by Region, 2022-2032 (US$ Mn)
8.7.2 Market Share by Region, 2022 & 2032 (%)
8.8 Lentivirus
8.8.1 Market Forecast by Region, 2022-2032 (US$ Mn)
8.8.2 Market Share by Region, 2022 & 2032 (%)
8.9 Others
8.9.1 Market Forecast by Region, 2022-2032 (US$ Mn)
8.9.2 Market Share by Region, 2022 & 2032 (%)
9 Viral Vectors and Plasmid DNA Manufacturing Market Analysis by Application
9.1 Key Findings
9.2 Application Segment: Market Attractiveness Index
9.3 Viral Vectors and Plasmid DNA Manufacturing Market Size Estimation and Forecast by Application
9.4 Antisense & RNAi
9.4.1 Market Forecast by Region, 2022-2032 (US$ Mn)
9.4.2 Market Share by Region, 2022 & 2032 (%)
9.5 Gene Therapy
9.5.1 Market Forecast by Region, 2022-2032 (US$ Mn)
9.5.2 Market Share by Region, 2022 & 2032 (%)
9.6 Cell Therapy
9.6.1 Market Forecast by Region, 2022-2032 (US$ Mn)
9.6.2 Market Share by Region, 2022 & 2032 (%)
9.7 Vaccinology
9.7.1 Market Forecast by Region, 2022-2032 (US$ Mn)
9.7.2 Market Share by Region, 2022 & 2032 (%)
10 Viral Vectors and Plasmid DNA Manufacturing Market Analysis by End-use
10.1 Key Findings
10.2 End-use Segment: Market Attractiveness Index
10.3 Viral Vectors and Plasmid DNA Manufacturing Market Size Estimation and Forecast by End-use
10.4 Pharma and Biopharma Companies
10.4.1 Market Forecast by Region, 2022-2032 (US$ Mn)
10.4.2 Market Share by Region, 2022 & 2032 (%)
10.5 Research Institutes
10.5.1 Market Forecast by Region, 2022-2032 (US$ Mn)
10.5.2 Market Share by Region, 2022 & 2032 (%)
11 Viral Vectors and Plasmid DNA Manufacturing Market Analysis by Disease
11.1 Key Findings
11.2 Disease Segment: Market Attractiveness Index
11.3 Viral Vectors and Plasmid DNA Manufacturing Market Size Estimation and Forecast by Disease
11.4 Oncology
11.4.1 Market Forecast by Region, 2022-2032 (US$ Mn)
11.4.2 Market Share by Region, 2022 & 2032 (%)
11.5 Genetic Disorders
11.5.1 Market Forecast by Region, 2022-2032 (US$ Mn)
11.5.2 Market Share by Region, 2022 & 2032 (%)
11.6 Infectious Diseases
11.6.1 Market Forecast by Region, 2022-2032 (US$ Mn)
11.6.2 Market Share by Region, 2022 & 2032 (%)
11.7 Others
11.7.1 Market Forecast by Region, 2022-2032 (US$ Mn)
11.7.2 Market Share by Region, 2022 & 2032 (%)
12 Viral Vectors and Plasmid DNA Manufacturing Market Analysis by Workflow
12.1 Key Findings
12.2 Workflow Segment: Market Attractiveness Index
12.3 Viral Vectors and Plasmid DNA Manufacturing Market Size Estimation and Forecast by Workflow
12.4 Upstream
12.4.1 Market Forecast by Region, 2022-2032 (US$ Mn)
12.4.2 Market Share by Region, 2022 & 2032 (%)
12.5 Downstream
12.5.1 Market Forecast by Region, 2022-2032 (US$ Mn)
12.5.2 Market Share by Region, 2022 & 2032 (%)
13 Viral Vectors and Plasmid DNA Manufacturing Market Analysis by Region
13.1 Key Findings
13.2 Regional Market Size Estimation and Forecast
14 North America Viral Vectors and Plasmid DNA Manufacturing Market Analysis
14.1 Key Findings
14.2 North America Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index
14.3 Market Size by Country, 2022, 2027 & 2032 (US$ Mn)
14.4 Market Size Estimation and Forecast by Country, 2022-2032 (US$ Mn)
14.5 Market Size Estimation and Forecast by Vector Type, 2022-2032 (US$ Mn)
14.6 Market Size Estimation and Forecast by Application, 2022-2032 (US$ Mn)
14.7 Market Size Estimation and Forecast by End-use, 2022-2032 (US$ Mn)
14.8 Market Size Estimation and Forecast by Disease, 2022-2032 (US$ Mn)
14.9 Market Size Estimation and Forecast by Workflow, 2022-2032 (US$ Mn)
14.10 U.S.
14.11 Canada
15 Europe Viral Vectors and Plasmid DNA Manufacturing Market Analysis
15.1 Key Findings
15.2 Europe Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index
15.3 Market Size by Country, 2022, 2027 & 2032 (US$ Mn)
15.4 Market Size Estimation and Forecast by Country, 2022-2032 (US$ Mn)
15.5 Market Size Estimation and Forecast by Vector Type, 2022-2032 (US$ Mn)
15.6 Market Size Estimation and Forecast by Application, 2022-2032 (US$ Mn)
15.7 Market Size Estimation and Forecast by End-use, 2022-2032 (US$ Mn)
15.8 Market Size Estimation and Forecast by Disease, 2022-2032 (US$ Mn)
15.9 Market Size Estimation and Forecast by Workflow, 2022-2032 (US$ Mn)
15.10 Germany
15.11 UK
15.12 France
15.13 Italy
15.14 Spain
15.15 Rest of Europe
16 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Analysis
16.1 Key Findings
16.2 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index
16.3 Market Size by Country, 2022, 2027 & 2032 (US$ Mn)
16.4 Market Size Estimation and Forecast by Country, 2022-2032 (US$ Mn)
16.5 Market Size Estimation and Forecast by Vector Type, 2022-2032 (US$ Mn)
16.6 Market Size Estimation and Forecast by Application, 2022-2032 (US$ Mn)
16.7 Market Size Estimation and Forecast by End-use, 2022-2032 (US$ Mn)
16.8 Market Size Estimation and Forecast by Disease, 2022-2032 (US$ Mn)
16.9 Market Size Estimation and Forecast by Workflow, 2022-2032 (US$ Mn)
16.10 Japan
16.11 China
16.12 India
16.13 Australia
16.14 South Korea
16.15 Rest of Asia Pacific
17 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Analysis
17.1 Key Findings
17.2 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index
17.3 Market Size by Country, 2022, 2027 & 2032 (US$ Mn)
17.4 Market Size Estimation and Forecast by Country, 2022-2032 (US$ Mn)
17.5 Market Size Estimation and Forecast by Vector Type, 2022-2032 (US$ Mn)
17.6 Market Size Estimation and Forecast by Application, 2022-2032 (US$ Mn)
17.7 Market Size Estimation and Forecast by End-use, 2022-2032 (US$ Mn)
17.8 Market Size Estimation and Forecast by Disease, 2022-2032 (US$ Mn)
17.9 Market Size Estimation and Forecast by Workflow, 2022-2032 (US$ Mn)
17.10 Brazil
17.11 Mexico
17.12 Argentina
17.13 Rest of Latin America
18 MEA Viral Vectors and Plasmid DNA Manufacturing Market Analysis
18.1 Key Findings
18.2 MEA Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index
18.3 Market Size by Country, 2022, 2027 & 2032 (US$ Mn)
18.4 Market Size Estimation and Forecast by Country, 2022-2032 (US$ Mn)
18.5 Market Size Estimation and Forecast by Vector Type, 2022-2032 (US$ Mn)
18.6 Market Size Estimation and Forecast by Application, 2022-2032 (US$ Mn)
18.7 Market Size Estimation and Forecast by End-use, 2022-2032 (US$ Mn)
18.8 Market Size Estimation and Forecast by Disease, 2022-2032 (US$ Mn)
18.9 Market Size Estimation and Forecast by Workflow, 2022-2032 (US$ Mn)
18.10 GCC
18.11 South Africa
18.12 Rest of MEA
19 Company Profiles
19.1 Strategic Outlook, 2019-2022
19.2 Merck KGaA
19.2.1 Company Snapshot
19.2.2 Company Overview
19.2.3 Financial Analysis, 2016-2021
19.2.3.1 Revenue, 2016-2021
19.2.3.2 Gross Profit, 2016-2021
19.2.3.3 R&D, 2016-2021
19.2.4 Product Benchmarking
19.2.5 Strategic Outlook
19.3 Lonza
19.3.1 Company Snapshot
19.3.2 Company Overview
19.3.3 Financial Analysis, 2016-2021
19.3.3.1 Revenue, 2016-2021
19.3.3.2 Gross Profit, 2016-2021
19.3.3.3 R&D, 2017-2020
19.3.4 Product Benchmarking
19.3.5 Strategic Outlook
19.4 FUJIFILM Diosynth Biotechnologies
19.4.1 Company Snapshot
19.4.2 Company Overview
19.4.3 Product Benchmarking
19.4.4 Strategic Outlook
19.5 Charles River Laboratories
19.5.1 Company Snapshot
19.5.2 Company Overview
19.5.3 Financial Analysis, 2016-2021
19.5.3.1 Revenue, 2016-2021
19.5.3.2 Gross Profit, 2016-2021
19.5.4 Product Benchmarking
19.5.5 Strategic Outlook
19.6 Patheon Pharma Services (Thermo Fisher Scientific Inc.)
19.6.1 Company Snapshot
19.6.2 Company Overview
19.6.3 Financial Analysis, 2016-2021
19.6.3.1 Revenue, 2016-2021
19.6.3.2 Gross Profit, 2016-2021
19.6.3.3 R&D, 2016-2021
19.6.4 Product Benchmarking
19.6.5 Strategic Outlook
19.7 Waisman Biomanufacturing
19.7.1 Company Snapshot
19.7.2 Company Overview
19.7.3 Product Benchmarking
19.8 Genezen
19.8.1 Company Snapshot
19.8.2 Company Overview
19.8.3 Product Benchmarking
19.8.4 Strategic Outlook
19.9 Yposkesi, Inc.
19.9.1 Company Snapshot
19.9.2 Company Overview
19.9.3 Product Benchmarking
19.9.4 Strategic Outlook
19.10 Advanced BioScience Laboratories, Inc. (ABL, Inc.)
19.10.1 Company Snapshot
19.10.2 Company Overview
19.10.3 Product Benchmarking
19.10.4 Strategic Outlook
19.11 Novasep (Thermofisher Scientific Inc.)
19.11.1 Company Snapshot
19.11.2 Company Overview
19.11.3 Product Benchmarking
19.11.4 Strategic Outlook
19.12 Cytiva
19.12.1 Company Snapshot
19.12.2 Company Overview
19.12.3 Product Benchmarking
19.12.4 Strategic Outlook
19.13 CEVEC Pharmaceuticals GmbH
19.13.1 Company Snapshot
19.13.2 Company Overview
19.13.3 Product Benchmarking
19.13.4 Strategic Outlook
19.14 Batavia Biosciences
19.14.1 Company Snapshot
19.14.2 Company Overview
19.14.3 Product Benchmarking
19.14.4 Strategic Outlook
19.15 Biovian Oy
19.15.1 Company Snapshot
19.15.2 Company Overview
19.15.3 Product Benchmarking
19.16 Wuxi AppTec
19.16.1 Company Snapshot
19.16.2 Company Overview
19.16.3 Financial Analysis, 2016-2021
19.16.3.1 Revenue, 2016-2021
19.16.3.2 Gross Profit, 2016-2020
19.16.4 Product Benchmarking
19.16.5 Strategic Outlook
19.17 VGXI, Inc.
19.17.1 Company Snapshot
19.17.2 Company Overview
19.17.3 Product Benchmarking
19.18 Paragon Bioservices, Inc. (Catalent)
19.18.1 Company Snapshot
19.18.2 Company Overview
19.18.3 Product Benchmarking
19.18.4 Strategic Outlook
19.19 Sirion-Biotech GmbH
19.19.1 Company Snapshot
19.19.2 Company Overview
19.19.3 Product Benchmarking
19.20 Virovek
19.20.1 Company Snapshot
19.20.2 Company Overview
19.20.3 Product Benchmarking
19.21 BioNTech IMFS
19.21.1 Company Snapshot
19.21.2 Company Overview
19.21.3 Product Benchmarking
19.22 Vivebiotech
19.22.1 Company Snapshot
19.22.2 Company Overview
19.22.3 Product Benchmarking
19.22.4 Strategic Outlook
19.23 Creative Biogene
19.23.1 Company Snapshot
19.23.2 Company Overview
19.23.3 Product Benchmarking
19.23.4 Strategic Outlook
19.24 Vibalogics
19.24.1 Company Snapshot
19.24.2 Company Overview
19.24.3 Product Benchmarking
19.24.4 Strategic Outlook
19.25 bluebird bio, Inc.
19.25.1 Company Snapshot
19.25.2 Company Overview
19.25.3 Financial Analysis, 2016-2021
19.25.3.1 Revenue, 2016-2021
19.25.3.2 R&D, 2016-2021
19.25.4 Product Benchmarking
19.25.5 Strategic Outlook
19.26 Aldevron
19.26.1 Company Snapshot
19.26.2 Company Overview
19.26.3 Product Benchmarking
20 Conclusion and Recommendations
20.1 Concluding Remarks from Visiongain
20.2 Recommendations for Market Players
List of Tables
Table 1 Viral Vectors and Plasmid DNA Manufacturing Market Snapshot, 2022 & 2032 (US$ million, CAGR %)
Table 2 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%): “V” Shaped Recovery
Table 3 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 4 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 5 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 6 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 7 Ongoing and Completed Clinical Trials Employing Ad Vectors
Table 8 Adenovirus Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 9 Retrovirus Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 10 Plasmid DNA Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 11 Ongoing Clinical Trials Employing AAV Vectors
Table 12 AAV Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 13 Lentivirus Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 14 Others Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 15 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 16 Antisense & RNAi Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 17 Gene Therapy Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 18 List of Cell Therapy Companies Worldwide by Location and Type
Table 19 Cell Therapy Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 20 Vaccinology Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 21 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-use, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 22 Pharma and Biopharma Companies Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 23 Research Institutes Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 24 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 25 Oncology Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 26 Genetic Disorders Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 27 Infectious Diseases Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 28 Others Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 29 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Workflow, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 30 Upstream Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 31 Downstream Segment Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 32 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 33 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 34 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 35 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 36 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-use, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 37 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 38 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Workflow, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 39 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 40 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 41 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 42 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 43 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 44 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-use, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 45 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 46 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Workflow, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 47 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 48 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 49 France Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 50 Italy Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 51 Spain Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 52 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 53 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 54 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 55 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 56 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-use, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 57 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 58 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Workflow, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 59 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 60 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 61 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 62 Australia Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 63 Australia Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 64 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 65 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 66 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 67 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 68 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-use, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 69 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 70 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Workflow, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 71 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 72 Mexico Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 73 Argentina Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 74 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 75 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 76 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 77 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 78 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-use, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 79 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 80 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Workflow, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 81 GCC Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 82 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 83 Rest of MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ mn, AGR%, CAGR%)
Table 84 Strategic Outlook, 2019-2022
Table 85 Merck KGaA: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 86 Merck KGaA: Product Portfolio
Table 87 Merck KGaA: Strategic Outlook
Table 88 Lonza: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 89 Lonza: Product Portfolio
Table 90 Lonza: Strategic Outlook
Table 91 FUJIFILM Diosynth Biotechnologies: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 92 FUJIFILM Diosynth Biotechnologies: Product Portfolio
Table 93 FUJIFILM Diosynth Biotechnologies: Strategic Outlook
Table 94 Charles River Laboratories: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 95 Charles River Laboratories: Product Portfolio
Table 96 Charles River Laboratories: Strategic Outlook
Table 97 Patheon Pharma Services (Thermo Fisher Scientific Inc.): Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 98 Patheon Pharma Services (Thermo Fisher Scientific Inc.): Product Portfolio
Table 99 Patheon Pharma Services (Thermo Fisher Scientific Inc.): Strategic Outlook
Table 100 Waisman Biomanufacturing: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 101 Waisman Biomanufacturing: Product Portfolio
Table 102 Genezen: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 103 Genezen: Product Portfolio
Table 104 Genezen: Strategic Outlook
Table 105 Yposkesi, Inc.: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 106 Yposkesi, Inc.: Product Portfolio
Table 107 Yposkesi, Inc.: Strategic Outlook
Table 108 Advanced BioScience Laboratories, Inc. (ABL, Inc.): Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 109 Advanced BioScience Laboratories, Inc. (ABL, Inc.): Product Portfolio
Table 110 Advanced BioScience Laboratories, Inc. (ABL, Inc.): Strategic Outlook
Table 111 Novasep: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 112 Novasep Holding S.A.S: Product Portfolio
Table 113 Novasep Holding S.A.S: Strategic Outlook
Table 114 Cytiva: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 115 Cytiva: Product Portfolio
Table 116 Cytiva: Strategic Outlook
Table 117 CEVEC Pharmaceuticals GmbH: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 118 CEVEC Pharmaceuticals GmbH: Product Portfolio
Table 119 CEVEC Pharmaceuticals GmbH: Strategic Outlook
Table 120 Batavia Biosciences: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 121 Batavia Biosciences: Product Portfolio
Table 122 Batavia Biosciences: Strategic Outlook
Table 123 Biovian Oy: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 124 Biovian Oy: Product Portfolio
Table 125 Wuxi AppTec: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 126 Wuxi AppTec: Product Portfolio
Table 127 Wuxi AppTec: Strategic Outlook
Table 128 VGXI, Inc.: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 129 VGXI, Inc.: Product Portfolio
Table 130 Paragon Bioservices, Inc.: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 131 Paragon Bioservices, Inc.: Product Portfolio
Table 132 Paragon Bioservices, Inc.: Strategic Outlook
Table 133 Sirion-Biotech GmbH: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 134 Sirion-Biotech GmbH: Product Portfolio
Table 135 Virovek : Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 136 Virovek : Product Portfolio
Table 137 BioNTech IMFS: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 138 BioNTech IMFS: Product Portfolio
Table 139 Vivebiotech: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 140 Vivebiotech: Product Portfolio
Table 141 Vivebiotech: Strategic Outlook
Table 142 Creative Biogene: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 143 Creative Biogene: Product Portfolio
Table 144 Creative Biogene: Strategic Outlook
Table 145 Vibalogics: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 146 Vibalogics: Product Portfolio
Table 147 Vibalogics: Strategic Outlook
Table 148 bluebird bio, Inc.: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 149 bluebird bio, Inc.: Product Portfolio
Table 150 bluebird bio, Inc.: Strategic Outlook
Table 151 Aldevron: Key Details (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 152 Aldevron: Product Portfolio
List of Figures
Figure 1 Viral Vectors and Plasmid DNA Manufacturing Market Segmentation
Figure 2 Viral Vectors and Plasmid DNA Manufacturing Market by Vector Type: Market Attractiveness Index
Figure 3 Viral Vectors and Plasmid DNA Manufacturing Market by Application: Market Attractiveness Index
Figure 4 Viral Vectors and Plasmid DNA Manufacturing Market by End-use: Market Attractiveness Index
Figure 5 Viral Vectors and Plasmid DNA Manufacturing Market by Disease: Market Attractiveness Index
Figure 6 Viral Vectors and Plasmid DNA Manufacturing Market by Workflow: Market Attractiveness Index
Figure 7 Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index by Region
Figure 8 Viral Vectors and Plasmid DNA Manufacturing Market: Market Dynamics
Figure 9 Cell and Gene Therapy Modalities
Figure 10 COVID Impact Analysis: Viral Vectors and Plasmid DNA Manufacturing Market Recovery Scenarios
Figure 11 Viral Vectors and Plasmid DNA Manufacturing Market by Region, 2022-2032 (US$ Mn, AGR %): “V” Shaped Recovery
Figure 12 Viral Vectors and Plasmid DNA Manufacturing Market by Region, 2022-2032 (US$ Mn, AGR %): “U” Shaped Recovery
Figure 13 Viral Vectors and Plasmid DNA Manufacturing Market by Region, 2022-2032 (US$ Mn, AGR %): “W” Shaped Recovery
Figure 14 Viral Vectors and Plasmid DNA Manufacturing Market by Region, 2022-2032 (US$ Mn, AGR %): “L” Shaped Recovery
Figure 15 Viral Vectors and Plasmid DNA Manufacturing Market: Porter’s Five Forces Analysis
Figure 16 Upstream Analysis
Figure 17 Downstream Analysis
Figure 18 Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index by Vector Type
Figure 19 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2022-2032 (US$ million)
Figure 20 Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Vector Type, 2022, 2027, 2032 (%)
Figure 21 Adenovirus Market Forecast by Region, 2022-2032 (US$ million)
Figure 22 Adenovirus Market Share Forecast by Region, 2022 & 2032 (%)
Figure 23 Retrovirus Market Forecast by Region, 2022-2032 (US$ million)
Figure 24 Retrovirus Market Share Forecast by Region, 2022 & 2032 (%)
Figure 25 Plasmid DNA Market Forecast by Region, 2022-2032 (US$ million)
Figure 26 Plasmid DNA Market Share Forecast by Region, 2022 & 2032 (%)
Figure 27 AAV Market Forecast by Region, 2022-2032 (US$ million)
Figure 28 AAV Market Share Forecast by Region, 2022 & 2032 (%)
Figure 29 Lentivirus Market Forecast by Region, 2022-2032 (US$ million)
Figure 30 Lentivirus Market Share Forecast by Region, 2022 & 2032 (%)
Figure 31 Others Market Forecast by Region, 2022-2032 (US$ million)
Figure 32 Others Market Share Forecast by Region, 2022 & 2032 (%)
Figure 33 Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index by Vector Type
Figure 34 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2022-2032 (US$ million)
Figure 35 Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Application, 2022, 2027, 2032 (%)
Figure 36 Antisense & RNAi Market Forecast by Region, 2022-2032 (US$ million)
Figure 37 Antisense & RNAi Market Share Forecast by Region, 2022 & 2032 (%)
Figure 38 Gene Therapy Market Forecast by Region, 2022-2032 (US$ million)
Figure 39 Gene Therapy Market Share Forecast by Region, 2022 & 2032 (%)
Figure 40 Cell Therapy Market Forecast by Region, 2022-2032 (US$ million)
Figure 41 Cell Therapy Market Share Forecast by Region, 2022 & 2032 (%)
Figure 42 Vaccinology Market Forecast by Region, 2022-2032 (US$ million)
Figure 43 Vaccinology Market Share Forecast by Region, 2022 & 2032 (%)
Figure 44 Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index by End-use
Figure 45 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-use, 2022-2032 (US$ million)
Figure 46 Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by End-use, 2022, 2027, 2032 (%)
Figure 47 Pharma and Biopharma Companies Market Forecast by Region, 2022-2032 (US$ million)
Figure 48 Pharma and Biopharma Companies Market Share Forecast by Region, 2022 & 2032 (%)
Figure 49 Research Institutes Market Forecast by Region, 2022-2032 (US$ million)
Figure 50 Research Institutes Market Share Forecast by Region, 2022 & 2032 (%)
Figure 51 Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index by Disease
Figure 52 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2022-2032 (US$ million)
Figure 53 Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Disease, 2022, 2027, 2032 (%)
Figure 54 Oncology Market Forecast by Region, 2022-2032 (US$ million)
Figure 55 Oncology Market Share Forecast by Region, 2022 & 2032 (%)
Figure 56 Genetic Disorders Market Forecast by Region, 2022-2032 (US$ million)
Figure 57 Genetic Disorders Market Share Forecast by Region, 2022 & 2032 (%)
Figure 58 Infectious Diseases Market Forecast by Region, 2022-2032 (US$ million)
Figure 59 Infectious Diseases Market Share Forecast by Region, 2022 & 2032 (%)
Figure 60 Others Market Forecast by Region, 2022-2032 (US$ million)
Figure 61 Others Market Share Forecast by Region, 2022 & 2032 (%)
Figure 62 Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index by Workflow
Figure 63 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Workflow, 2022-2032 (US$ million)
Figure 64 Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Workflow, 2022, 2027, 2032 (%)
Figure 65 Upstream Market Forecast by Region, 2022-2032 (US$ million)
Figure 66 Upstream Market Share Forecast by Region, 2022 & 2032 (%)
Figure 67 Downstream Market Forecast by Region, 2022-2032 (US$ million)
Figure 68 Downstream Market Share Forecast by Region, 2022 & 2032 (%)
Figure 69 Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region 2022, 2032 (Revenue, CAGR%)
Figure 70 Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Region 2022, 2027, 2032(%)
Figure 71 Viral Vectors and Plasmid DNA Manufacturing Market by Region, 2022-2032 (US$ Mn)
Figure 72 North America Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index
Figure 73 North America Viral Vectors and Plasmid DNA Manufacturing Market by Region, 2022, 2027 & 2032 (US$ million)
Figure 74 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2022-2032 (US$ million)
Figure 75 North America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Country, 2022 & 2032 (%)
Figure 76 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2022-2032 (US$ million)
Figure 77 North America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Vector Type, 2022 & 2032 (%)
Figure 78 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2022-2032 (US$ million)
Figure 79 North America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Application, 2022 & 2032 (%)
Figure 80 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-use, 2022-2032 (US$ million)
Figure 81 North America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by End-use, 2022 & 2032 (%)
Figure 82 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2022-2032 (US$ million)
Figure 83 North America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Disease, 2022 & 2032 (%)
Figure 84 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Workflow, 2022-2032 (US$ million)
Figure 85 North America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Workflow, 2022 & 2032 (%)
Figure 86 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 87 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 88 Europe Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index
Figure 89 Europe Viral Vectors and Plasmid DNA Manufacturing Market by Region, 2022, 2027 & 2032 (US$ million)
Figure 90 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2022-2032 (US$ million)
Figure 91 Europe Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Country, 2022 & 2032 (%)
Figure 92 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2022-2032 (US$ million)
Figure 93 Europe Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Vector Type, 2022 & 2032 (%)
Figure 94 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2022-2032 (US$ million)
Figure 95 Europe Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Application, 2022 & 2032 (%)
Figure 96 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-use, 2022-2032 (US$ million)
Figure 97 Europe Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by End-use, 2022 & 2032 (%)
Figure 98 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2022-2032 (US$ million)
Figure 99 Europe Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Disease, 2022 & 2032 (%)
Figure 100 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Workflow, 2022-2032 (US$ million)
Figure 101 Europe Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Workflow, 2022 & 2032 (%)
Figure 102 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 103 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 104 France Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 105 Italy Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 106 Spain Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 107 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 108 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index
Figure 109 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market by Region, 2022, 2027 & 2032 (US$ million)
Figure 110 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2022-2032 (US$ million)
Figure 111 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Country, 2022 & 2032 (%)
Figure 112 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2022-2032 (US$ million)
Figure 113 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Vector Type, 2022 & 2032 (%)
Figure 114 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2022-2032 (US$ million)
Figure 115 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Application, 2022 & 2032 (%)
Figure 116 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-use, 2022-2032 (US$ million)
Figure 117 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by End-use, 2022 & 2032 (%)
Figure 118 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2022-2032 (US$ million)
Figure 119 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Disease, 2022 & 2032 (%)
Figure 120 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Workflow, 2022-2032 (US$ million)
Figure 121 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Workflow, 2022 & 2032 (%)
Figure 122 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 123 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 124 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 125 Australia Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 126 South Korea Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 127 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 128 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index
Figure 129 Latin America Viral Vectors and Plasmid DNA Manufacturing Market by Region, 2022, 2027 & 2032 (US$ million)
Figure 130 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2022-2032 (US$ million)
Figure 131 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Country, 2022 & 2032 (%)
Figure 132 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2022-2032 (US$ million)
Figure 133 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Vector Type, 2022 & 2032 (%)
Figure 134 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2022-2032 (US$ million)
Figure 135 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Application, 2022 & 2032 (%)
Figure 136 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-use, 2022-2032 (US$ million)
Figure 137 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by End-use, 2022 & 2032 (%)
Figure 138 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2022-2032 (US$ million)
Figure 139 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Disease, 2022 & 2032 (%)
Figure 140 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Workflow, 2022-2032 (US$ million)
Figure 141 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Workflow, 2022 & 2032 (%)
Figure 142 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 143 Mexico Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million), AGR %
Figure 144 Argentina Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 145 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 146 MEA Viral Vectors and Plasmid DNA Manufacturing Market Attractiveness Index
Figure 147 MEA Viral Vectors and Plasmid DNA Manufacturing Market by Region, 2022, 2027 & 2032 (US$ million)
Figure 148 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2022-2032 (US$ million)
Figure 149 MEA Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Country, 2022 & 2032 (%)
Figure 150 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2022-2032 (US$ million)
Figure 151 MEA Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Vector Type, 2022 & 2032 (%)
Figure 152 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2022-2032 (US$ million)
Figure 153 MEA Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Application, 2022 & 2032 (%)
Figure 154 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-use, 2022-2032 (US$ million)
Figure 155 MEA Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by End-use, 2022 & 2032 (%)
Figure 156 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2022-2032 (US$ million)
Figure 157 MEA Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Disease, 2022 & 2032 (%)
Figure 158 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Workflow, 2022-2032 (US$ million)
Figure 159 MEA Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Workflow, 2022 & 2032 (%)
Figure 160 GCC Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
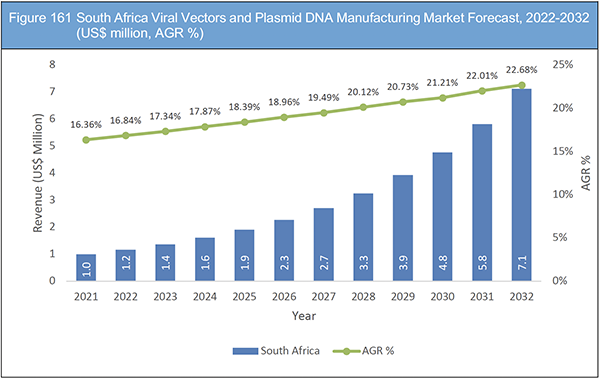
Figure 161 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 162 Rest of MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2022-2032 (US$ million, AGR %)
Figure 163 Merck KGaA.: Net Revenue, 2016-2021 (US$ Mn, AGR%)
Figure 164 Merck KGaA.: Gross Profit, 2016-2021 (US$ Mn, AGR%)
Figure 165 Merck KGaA.: R&D, 2016-2021 (US$ Mn, AGR%)
Figure 166 Lonza.: Net Revenue, 2016-2021 (US$ Mn, AGR%)
Figure 167 Lonza.: Gross Profit, 2016-2021 (US$ Mn, AGR%)
Figure 168 Lonza.: R&D, 2017-2020 (US$ Mn, AGR%)
Figure 169 Charles River Laboratories.: Net Revenue, 2016-2021 (US$ Mn, AGR%)
Figure 170 Charles River Laboratories.: Gross Profit, 2016-2021 (US$ Mn, AGR%)
Figure 171 Thermofisher Fisher Scientific Inc..: Net Revenue, 2016-2021 (US$ Mn, AGR%)
Figure 172 Thermofisher Scientific Inc..: Gross Profit, 2016-2021 (US$ Mn, AGR%)
Figure 173 Thermofisher Scientific Inc.: R&D, 2016-2021 (US$ Mn, AGR%)
Figure 174 Wuxi AppTec.: Net Revenue, 2016-2021 (US$ Mn, AGR%)
Figure 175 Wuxi AppTec.: Gross Profit, 2016-2020 (US$ Mn, AGR%)
Figure 176 bluebird bio, Inc.: Net Revenue, 2016-2021 (US$ Mn, AGR%)
Figure 177 bluebird bio, Inc.: R&D, 2016-2021 (US$ Mn, AGR%)
Advanced BioScience Laboratories, Inc. (ABL, Inc.)
Batavia Biosciences
BioMarin
BioNTech IMFS
Biovian Oy
bluebird bio, Inc.
CEVEC Pharmaceuticals GmbH
Charles River Laboratories
Creative Biogene
Cytiva
FUJIFILM Diosynth Biotechnologies
Genezen
Lonza
Merck KGaA
Novasep (Thermofisher Scientific Inc.)
Paragon Bioservices, Inc. (Catalent)
Patheon Pharma Services (Thermo Fisher Scientific Inc.)
Sirion-Biotech GmbH
VGXI, Inc.
Vibalogics
Virovek
Vivebiotech
Waisman Biomanufacturing
Wuxi AppTec, Yposkesi, Inc.
Yposkesi, Inc.
List of Other Companies Mentioned in the Report
Abeona Therapeutics
Acucela
Adaptimmune Therapeutics
Autolus
AveXis
BioCancell
Biomay
Biomiga
BioReliance
Biotec Services International
Celladon
Cellectis
Cellular Biomedicine Group
Delphi Genetics
Department of Neuroscience, University of Minnesota
Desktop Genetics
DNAtrix
Elixirgen Scientific
Epeius Biotechnologies
EUFETS
Eurofins Genomics
GEG Tech
Genable Technologies
Immune Design
Immune Technology
ImmunoGenes
Immunomic Therapeutics
Inbiomed
Indiana University Vector Production Facility
Voyager Therapeutics
Xpress Biologics
List of Associations Mentioned in the Report
Alliance for Regenerative Medicine
European Society of Gene and Cell Therapy
Gene Therapy Net
Informa UK Limited
IntechOpen
JOURNAL OF GENERAL VIROLOGY
National Center for Biotechnology Information
Pan European Networks Ltd
Pharma Manufacturing
Pharmaceutical-Networking.com
The Royal Society
Transgene
University of Rochester
Download sample pages
Complete the form below to download your free sample pages for Viral Vectors & Plasmid DNA Manufacturing Market Report 2022-2032
Related reports
-

mRNA Vaccines & Therapeutics Market Report 2022-2032
The global mRNA Vaccines & Therapeutics market was valued at US$58,452.6 million in 2021 and is projected to change at...Full DetailsPublished: 30 August 2022 -

Gene Therapy R&D Market Report 2022-2032
The gene therapy R&D market was valued at US$1,653.0 million in 2021 and is projected to grow at a CAGR...Full DetailsPublished: 15 September 2022 -

Contract Research Organisations (CROs) Market Report 2022-2032
The global Contract Research Organizations (CROs) market was valued at US$57.1 billion in 2021 and is projected to grow at...Full DetailsPublished: 12 August 2022
Download sample pages
Complete the form below to download your free sample pages for Viral Vectors & Plasmid DNA Manufacturing Market Report 2022-2032
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Don’t Miss Out!
Subscribe to receive the latest Industry news, trending insight and analysis straight to your inbox.Choose your preferences:
Latest Pharma news
Retinal Gene Therapy Market
The global Retinal Gene Therapy market is projected to grow at a CAGR of 9.6% by 2034
26 July 2024
HIV Drugs and Injectables Market
The global HIV Drugs & Injectables market is projected to grow at a CAGR of 4.6 % by 2034
24 July 2024
Digital Twin Technology in Pharmaceutical Manufacturing Market
The global Digital Twin Technology in Pharmaceutical Manufacturing market is projected to grow at a CAGR of 31.3% by 2034
23 July 2024
Specialty Pharma Market
The global Specialty Pharma market is projected to grow at a CAGR of 7.5% by 2034
22 July 2024


















