Industries > Pharma > Clinical Trial Supply and Logistics Market for Pharma 2020-2030
Clinical Trial Supply and Logistics Market for Pharma 2020-2030
Forecasts by Type (Clinical Trial Manufacturing (Clinical Trial Packaging, Others), Clinical Trial Logistics and Distribution (Clinical Trial Cold Chain Logistics, Others), Clinical Trial Supply Chain Management), by End Users (CRO’s, Pharma & Biotech, Others (Academics, Research Institutes etc.)) PLUS COVID-19 Impact Analysis, and leading companies analysis in the Clinical Trial Supply and Logistics Market
The global clinical trials supply and logistics market for pharma is estimated to have reach $10.3bn in 2019, dominated by the clinical trial manufacturing segment. The global clinical trial supply and logistics market for pharma is expected to grow at a CAGR of 6.6% in the first half of the forecast period. This new report also covers the economic impact of COVID-19 as well as how the pandemic will affect your industry sector.
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in this sector.
In this brand new 306-page report you will receive 90 charts– all unavailable elsewhere.
The 306-page report provides clear detailed insight into the global clinical trial supply and logistics market for pharma. Discover the key drivers and challenges affecting the market.
The report also includes insight on how COVID-19 will affect your industry.
By ordering and reading our brand-new report today you stay better informed and ready to act.
Report Scope
• Revenue and growth forecasts from 2020 to 2030 for the global clinical trial supply and logistics market. This report also discusses the Drivers and Restraints of the global clinical trial supply and logistics market.
• Revenue and growth forecasts from 2020 to 2030 for the leading submarkets of the global clinical trial supply and logistics market:
• Manufacturing – also with sub forecasting for packaging and for other production
• Logistics and distribution – also with sub forecasts for cold chain logistics and for other services
• Supply chain management
This report discusses the Drivers and Restraints of each submarket.
• Revenue and growth forecasts from 2019 to 2029 for the leading national markets:
• United States
• Japan
• EU5: Germany, France, United Kingdom, Italy, Spain
• Brazil
• Mexico
• China
• India
• GCC
• South Africa
• Rest of the World
Revenues forecast for the US, Japan, EU5, Brazil, Mexico, China, India, GCC, South Africa are further broken down by submarket.
• This report profiles the leading companies offering clinical trial supply and logistics services to the pharmaceutical industry:
• ADAllen Pharma
• Almac Group
• Amatsigroup
• Catalent
• DHL
• FedEx
• Fisher Clinical Services
• Marken
• Parexel International
• Sharp Packaging Services, LLC
• Many others
• This report provides qualitative analysis of the clinical trial supply and logistics market. This report discusses the Strengths, Weaknesses, Opportunities and Threats of the clinical trial supply and logistics market. Social, Technological, Economic and Political factors that influence this market are also discussed.
• This report discusses trends in the clinical trial supply and logistics market, clinical trial manufacturing market, comparator sourcing, clinical trial packaging, clinical trial supply chain management.
• This report discusses the regulatory outlook of the clinical trial supply and logistics industry, outlook for cold chain logistics in the clinical trial sector, as well as regulatory aspects of cold chain distribution for clinical trial materials
• Key Questions Answered by this Report:
• How is the clinical trial supply and logistics market for pharma evolving?
• What are the drivers and restraints for the growth of the clinical trial supply and logistics market for pharma?
• How will each clinical trial supply and logistics submarket for pharma grow over the forecast period and how much revenue will these submarkets account for in 2030?
• How will the market shares for each clinical trial supply and logistics submarket for pharma develop to 2030?
• What is the value of the leading clinical trial supply and logistics submarket for pharma s in important regions of the world?
• What will be the main driver for the overall market to 2030?
• How will the market shares of the national markets change by 2030 and which geographical region will lead the market by 2030?
• Who are the leading players and what are their prospects over the forecast period?
Visiongain’s study is intended for anyone requiring commercial analyses for the global clinical trial supply and logistics market for pharma. You find data, trends and predictions.
Buy our report today Clinical Trial Supply and Logistics Market for Pharma: Forecasts by Type (Clinical Trial Manufacturing (Clinical Trial Packaging, Others), Clinical Trial Logistics and Distribution (Clinical Trial Cold Chain Logistics, Others), Clinical Trial Supply Chain Management), by End Users (CRO’s, Pharma & Biotech, Others (Academics, Research Institutes etc.)) PLUS COVID-19 Impact Analysis.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: sara.peerun@visiongain.com
1 Executive Summary
1.1 Why You Should Read This Report
1.2 What This Report Delivers
1.3 Key Questions Answered by This Analytical Report Include:
1.4 Who is This Report For?
1.5 Methodology
1.6 Frequently Asked Questions (FAQs)
1.7 Associated Visiongain Reports
1.8 About Visiongain
2 Introduction to the Clinical Trial Supply and Logistics Market
2.1 Clinical Trial Supply and Logistics Market Definition
2.2 Clinical Trial Supply and Logistics Market Segmentation
2.3 Clinical Trials Are Becoming Increasingly Complex
2.4 Clinical Trial Globalisation: Access to New Markets and New Patients
2.5 Outsourcing Clinical Trial Supply and Logistics
2.6 What Services Are Most Commonly Outsourced?
2.7 Which Companies Participate in the Clinical Trial Supply and Logistics Market?
2.8 Clinical Trial Supply and Logistics: Trends and Future Outlook
3 Global Clinical Trial Supply and Logistics Market Overview
3.1 Drivers
3.1.1 Growing Number of Clinical Trials
3.1.2 Clinical Trial Globalisation
3.1.3 Technological Advancements
3.1.4 Growing Focus on Outsourcing
3.2 Restraints
3.3 SWOT Analysis
3.4 Industry analysis – PEST
3.4.1 Political
3.4.2 Economic
3.4.3 Social
3.4.4 Technological
4 Economic Impact of COVID-19
4.1 Global growth
4.2 Global Trade
4.3 Pharmaceutical Impact
4.4 Clinical Trial Impact
5 Regulatory Outlook
5.1 How do Regulatory Standards Affect Clinical Trial Supply?
5.2 Good Practices (GxP) in Clinical Trial Distribution
5.3 Good Manufacturing Practice (GMP)
5.4 Good Distribution Practice (GDP)
5.5 Import Regulations Complicate International Manufacturing and Trials
5.6 Clinical Trial Supply Regulations in the US 2018
5.7 Importing Drugs into the US: A Reinterpretation of the Rules
5.8 Clinical Trial Regulations in the EU 2018
5.9 Manufacturing Requirements for Clinical Trial Materials in the EU
5.10 The European Commission Releases New GDP Guidelines 2013
5.11 GMP and Importation Regulations for Pharmaceuticals in Japan 2017
5.12 Clinical Trial Supply Chain Regulations in Emerging Markets
5.13 China: Tougher Regulations for Pharmaceutical Companies and Service Providers
5.14 Improving Supply Chain Security in China 2018
5.15 India: Drug Manufacturing and Imports for Clinical Trials
5.16 The CDSCO Drafts New GDP Guidelines
5.17 Brazil: Slow Regulatory Approval for Clinical Trials
5.18 Recent Updates to Pharmaceutical Regulation in Russia
6 Global Clinical Trial Supply and Logistics Market Size Forecast 2020-2030 by Type
6.1 Clinical Trial Manufacturing Forecast 2020-2030
6.2 Clinical Trial Manufacturing Market by Type (Clinical Trial Packaging, Others), Forecast 2020-2030
6.3 “V” Shaped Recovery
6.4 “U” Shaped Recovery
6.5 “W” Shaped Recovery
6.6 “L” Shaped Recovery
6.7 Clinical Trial Logistics and Distribution Forecast 2020-2030
6.8 Clinical Trial Logistics & Distribution Market by Type (Clinical Trial Cold Chain Logistics, Others), Forecast 2020-2030
6.9 “V” Shaped Recovery
6.10 “U” Shaped Recovery
6.11 “W” Shaped Recovery
6.12 “L” Shaped Recovery
6.13 Clinical Trial Supply Chain Management Forecast 2020-2030
6.14 “V” Shaped Recovery
6.15 “U” Shaped Recovery
6.16 “W” Shaped Recovery
6.17 “L” Shaped Recovery
7 Global Clinical Trial Supply and Logistics Market Size Forecast 2020-2030 by End Users
7.1 CRO’s Forecast 2020-2030
7.1.1 CRO’s Market Forecast by Region, 2020-2030
7.2 “V” Shaped Recovery
7.3 “U” Shaped Recovery
7.4 “W” Shaped Recovery
7.5 “L” Shaped Recovery
7.6 Pharma and Biotech Forecast 2020-2030
7.6.1 Pharma & Biotech Market Forecast by Region, 2020-2030
7.7 “V” Shaped Recovery
7.8 “U” Shaped Recovery
7.9 “W” Shaped Recovery
7.10 “L” Shaped Recovery
7.11 Others Forecast 2020-2030
7.11.1 Other End Users Market Forecast by Region, 2020-2030
7.12 “V” Shaped Recovery
7.13 “U” Shaped Recovery
7.14 “W” Shaped Recovery
7.15 “L” Shaped Recovery
8 Regional and Leading National Clinical Trial Supply and Logistics Market Forecasts 2020-2030
8.1 Global Clinical Trial Supply and Logistics Market by National Market Share Forecast 2020-2030
8.2 North America Clinical Trial Supply and Logistics Market
8.2.1 North America Clinical Trial Supply and Logistics Market by Country Market Share Forecast 2020-2030
8.2.2 North America Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030
8.2.2.1 North America Clinical Trial Manufacturing Market by Type (Clinical Trial Packaging, Others), Forecast 2020-2030
8.2.2.2 North America Clinical Trial Logistics & Distribution Market by Type (Clinical Trial Cold Chain Logistics, Others), Forecast 2020-2030
8.2.3 North America Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030
8.2.4 U.S. Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.3 “V” Shaped Recovery
8.4 “U” Shaped Recovery
8.5 “W” Shaped Recovery
8.6 “L” Shaped Recovery
8.6.1 Canada Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.7 “V” Shaped Recovery
8.8 “U” Shaped Recovery
8.9 “W” Shaped Recovery
8.10 “L” Shaped Recovery
8.11 Europe Clinical Trial Supply and Logistics Market
8.11.1 Europe Clinical Trial Supply and Logistics Market by Country Market Share Forecast 2020-2030
8.11.2 Europe Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030
8.11.2.1 Europe Clinical Trial Manufacturing Market by Type (Clinical Trial Packaging, Others), Forecast 2020-2030
8.11.2.2 Europe Clinical Trial Logistics & Distribution Market by Type(Clinical Trial Cold Chain Logistics, Others), Forecast 2020-2030
8.11.3 Europe Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030
8.11.4 Germany Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.12 “V” Shaped Recovery
8.13 “U” Shaped Recovery
8.14 “W” Shaped Recovery
8.15 “L” Shaped Recovery
8.15.1 France Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.16 “V” Shaped Recovery
8.17 “U” Shaped Recovery
8.18 “W” Shaped Recovery
8.19 “L” Shaped Recovery
8.19.1 UK Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.20 “V” Shaped Recovery
8.21 “U” Shaped Recovery
8.22 “W” Shaped Recovery
8.23 “L” Shaped Recovery
8.23.1 Italy Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.24 “V” Shaped Recovery
8.25 “U” Shaped Recovery
8.26 “W” Shaped Recovery
8.27 “L” Shaped Recovery
8.27.1 Spain Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.28 “V” Shaped Recovery
8.29 “U” Shaped Recovery
8.30 “W” Shaped Recovery
8.31 “L” Shaped Recovery
8.31.1 Rest of Europe Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.32 “V” Shaped Recovery
8.33 “U” Shaped Recovery
8.34 “W” Shaped Recovery
8.35 “L” Shaped Recovery
8.36 Asia Pacific Clinical Trial Supply and Logistics Market by Country Market Share Forecast 2020-2030
8.36.1 Asia Pacific Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030
8.36.1.1 Asia Pacific Clinical Trial Manufacturing Market by Type (Clinical Trial Packaging, Others), Forecast 2020-2030
8.36.1.2 Asia Pacific Clinical Trial Logistics & Distribution Market by Type (Clinical Trial Cold Chain Logistics, Others), Forecast 2020-2030
8.36.2 Asia Pacific Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030
8.36.3 Japan Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.37 “V” Shaped Recovery
8.38 “U” Shaped Recovery
8.39 “W” Shaped Recovery
8.40 “L” Shaped Recovery
8.40.1 China Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.41 “V” Shaped Recovery
8.42 “U” Shaped Recovery
8.43 “W” Shaped Recovery
8.44 “L” Shaped Recovery
8.44.1 India Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.45 “V” Shaped Recovery
8.46 “U” Shaped Recovery
8.47 “W” Shaped Recovery
8.48 “L” Shaped Recovery
8.48.1 ASEAN Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.49 “V” Shaped Recovery
8.50 “U” Shaped Recovery
8.51 “W” Shaped Recovery
8.52 “L” Shaped Recovery
8.52.1 Rest of Asia Pacific Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.53 “V” Shaped Recovery
8.54 “U” Shaped Recovery
8.55 “W” Shaped Recovery
8.56 “L” Shaped Recovery
8.57 Latin America Clinical Trial Supply and Logistics Market by Country Market Share Forecast 2020-2030
8.57.1 Latin America Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030
8.57.1.1 Latin America Clinical Trial Manufacturing Market by Type (Clinical Trial Packaging, Others), Forecast 2020-2030
8.57.1.2 Latin America Clinical Trial Logistics & Distribution Market by Type (Clinical Trial Cold Chain Logistics, Others), Forecast 2020-2030
8.57.2 Latin America Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030
8.57.3 Brazil Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.58 “V” Shaped Recovery
8.59 “U” Shaped Recovery
8.60 “W” Shaped Recovery
8.61 “L” Shaped Recovery
8.61.1 Mexico Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.62 “V” Shaped Recovery
8.63 “U” Shaped Recovery
8.64 “W” Shaped Recovery
8.65 “L” Shaped Recovery
8.65.1 Rest of Latin America Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.66 “V” Shaped Recovery
8.67 “U” Shaped Recovery
8.68 “W” Shaped Recovery
8.69 “L” Shaped Recovery
8.70 Middle East and Africa Clinical Trial Supply and Logistics Market by Country Market Share Forecast 2020-2030
8.70.1 Middle East and Africa Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030
8.70.1.1 MEA Clinical Trial Manufacturing Market by Type (Clinical Trial Packaging, Others), Forecast 2020-2030
8.70.1.2 MEA Clinical Trial Logistics & Distribution Market by Type (Clinical Trial Cold Chain Logistics, Others), Forecast 2020-2030
8.70.2 Middle East and Africa Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030
8.70.3 GCC Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.71 “V” Shaped Recovery
8.72 “U” Shaped Recovery
8.73 “W” Shaped Recovery
8.74 “L” Shaped Recovery
8.74.1 South Africa Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.75 “V” Shaped Recovery
8.76 “U” Shaped Recovery
8.77 “W” Shaped Recovery
8.78 “L” Shaped Recovery
8.78.1 Rest of Middle East and Africa Clinical Trial Supply and Logistics Market Forecast 2020-2030
8.79 “V” Shaped Recovery
8.80 “U” Shaped Recovery
8.81 “W” Shaped Recovery
8.82 “L” Shaped Recovery
9 Leading Companies in the Clinical Trial Supply and Logistics Market
9.1 AmerisourceBergen Corporation
9.1.1 AmerisourceBergen Corporation: Company Snapshot
9.1.2 AmerisourceBergen Corporation: Company Overview
9.1.3 AmerisourceBergen Corporation Total Company Sales 2016-2019
9.1.4 AmerisourceBergen Corporation Clinical Trial Supply and Logistics Products / Services
9.1.5 AmerisourceBergen Corporation. Mergers & Acquisitions (M&A) Activity
9.2 DHL
9.2.1 DHL: Company Snapshot
9.2.2 DHL: Company Overview
9.2.3 DHL Total Company Sales 2016-2019
9.2.4 DHL Clinical Trial Supply and Logistics Products / Services
9.2.5 DHL. Mergers & Acquisitions (M&A) Activity
9.3 MARKEN
9.3.1 MARKEN: Company Snapshot
9.3.2 MARKEN: Company Overview
9.3.3 MARKEN Total Company Sales 2016-2019
9.3.4 MARKEN Clinical Trials Supply and Logistics Products / Services
9.3.5 MARKEN. Mergers & Acquisitions (M&A) Activity
9.4 Fisher Clinical Services, Inc.
9.4.1 Fisher Clinical Services: Company Snapshot
9.4.2 Fisher Clinical Services Inc.: Company Overview
9.4.3 Fisher Clinical Services, Inc. Total Company Sales 2016-2019
9.4.4 Fisher Clinical Services, Inc. Clinical Trials Supply and Logistics Products / Services
9.5 Ancillare, LP
9.5.1 Ancillare, LP: Company Snapshot
9.5.2 Ancillare, LP: Company Overview
9.5.3 Ancillare, LP Clinical Trials Supply and Logistics Products / Services
9.5.4 Ancillare, LP. Mergers & Acquisitions (M&A) Activity
9.6 Sharp
9.6.1 Sharp: Company Snapshot
9.6.2 Sharp: Company Overview
9.6.3 Sharp Clinical Trials Supply and Logistics Products / Services
9.6.4 Sharp. Mergers & Acquisitions (M&A) Activity
9.7 Endpoint Clinical, Inc.
9.7.1 Endpoint Clinical, Inc.: Company Snapshot
9.7.2 Endpoint Clinical, Inc.: Company Overview
9.7.3 Endpoint Clinical, Inc. Clinical Trials Supply and Logistics Products / Services
9.7.4 Endpoint Clinical, Inc.. Mergers & Acquisitions (M&A) Activity
9.8 Parexel International Corporation
9.8.1 Parexel International Corporation: Company Snapshot
9.8.2 Parexel International Corporation: Company Overview
9.8.3 PAREXEL International Corporation Total Company Sales 2016-2019
9.8.4 PAREXEL International Corporation Clinical Trials Supply and Logistics Products / Services
9.8.5 PAREXEL International Corporation Mergers & Acquisitions (M&A) Activity
9.9 PRA Health Sciences
9.9.1 PRA Health Sciences: Company Snapshot
9.9.2 PRA Health Sciences: Company Overview
9.9.3 PRA Health Sciences Total Company Sales 2016-2019
9.9.4 PRA Health Sciences Clinical Trials Supply and Logistics Products / Services
9.9.5 PRA Health Sciences. Mergers & Acquisitions (M&A) Activity
9.10 Almac Group
9.10.1 Almac Group: Company Snapshot
9.10.2 Almac Group: Company Overview
9.10.3 Almac Group Clinical Trials Supply and Logistics Products / Services
9.10.4 Almac Group. Mergers & Acquisitions (M&A) Activity
9.11 Catalent, Inc.
9.11.1 Catalent, Inc.: Company Snapshot
9.11.2 Catalent, Inc.: Company Overview
9.11.3 Catalent, Inc. Total Company Sales 2016-2019
9.11.4 Catalent, Inc. Clinical Trials Supply and Logistics Products / Services
9.11.5 Catalent, Inc. Mergers & Acquisitions (M&A) Activity
9.12 The Coghlan Group
9.12.1 The Coghlan Group: Company Overview
9.12.2 The Coghlan Group Clinical Trials Supply and Logistics Products / Services
9.12.3 The Coghlan Group Mergers & Acquisitions (M&A) Activity
9.13 FedEX
9.13.1 FedEX: Company Snapshot
9.13.2 FedEX: Company Overview
9.13.3 FedEX Total Company Sales 2016-2019
9.13.4 FedEX Clinical Trials Supply and Logistics Products / Services
9.13.5 FedEX Mergers & Acquisitions (M&A) Activity
10 Conclusion
10.1 Impact of COVID-19 on Global Clinical Trial Supply and Logistics Market
10.2 Clinical Trial Supply Chain Management Trends to 2030
10.3 Forecasting and Modelling to Ensure Sufficient Supply
10.4 IT Systems Improve Clinical Trial Planning: IVRS and IWRS
10.5 Home-Based Trial Participation: Opportunities and Challenges for Logistics
10.6 How Will Greater Use of Adaptive Clinical Trials Impact Trial Logistics?
10.7 Outsourcing Clinical Trial Supply and Logistics
10.8 Managing Multiple Supplier Networks
Associated Visiongain Reports
Visiongain Report Sales Order Form
Appendix A
About Visiongain
Appendix B
Visiongain report evaluation form
List of Figures
Figure 1. Global Clinical Trial Supply and Logistics Market Forecast by Region, 2020-2030 (US$ billion, AGR %)
Figure 2. Global Clinical Trial Supply and Logistics Market Share Forecast by Region, 2020, 2025, 2030 (%)
Figure 3. Global Clinical Trial Supply and Logistics Market Segmentation
Figure 4. Number of Registered Studies and Percentage of Total (as of April 10, 2020)
Figure 5. Trends Affecting the Clinical Trial Supply and Logistics Market, 2020-2030
Figure 6. Global Clinical Trial Supply and Logistics Market Drivers
Figure 7. Global Clinical Trial Supply and Logistics Market Restraints
Figure 8. Strengths and Weaknesses
Figure 9. Opportunities and Threats
Figure 10. Export Trade Volume (2018 & 2019: Historical; 2020 & 2021: Optimistic Scenario;
Figure 11. Import Trade Volume (2018 & 2019: Historical; 2020 & 2021: Optimistic Scenario;
Figure 12. Real GDP at market exchange rates
Figure 13. Global Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 14. Global Clinical Trial Supply and Logistics Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 15. Clinical Trial Manufacturing Revenue Forecast 2020-2030 (US$ billion, AGR%)
Figure 16. Global Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 17. Global Clinical Trial Manufacturing Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 18. Clinical Trial Manufacturing Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 19. Clinical Trial Manufacturing Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 20. Clinical Trial Manufacturing Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 21. Clinical Trial Manufacturing Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 22. Clinical Trial Logistics and Distribution: Drivers & Restraints
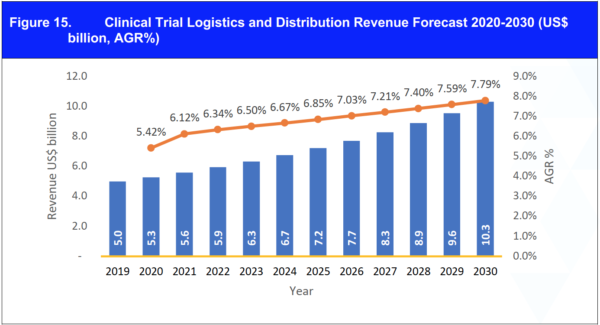
Figure 23. Clinical Trial Logistics and Distribution Revenue Forecast 2020-2030 (US$ billion, AGR%)
Figure 24. Global Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 25. Global Clinical Trial Logistics & Distribution Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 26. Clinical Trial Logistics & Distribution Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 27. Clinical Trial Logistics & Distribution Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 28. Clinical Trial Logistics & Distribution Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 29. Clinical Trial Logistics & Distribution Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 30. Clinical Trial Supply Chain Management Drivers & Restraints
Figure 31. Clinical Trial Supply Chain Management Revenue Forecast 2020-2030 (US$ billion, AGR%)
Figure 32. Clinical Trial Supply Chain Management Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 33. Clinical Trial Supply Chain Management Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 34. Clinical Trial Supply Chain Management Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 35. Clinical Trial Supply Chain Management Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 36. Global Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030 (US$ billion, AGR %)
Figure 37. Global Clinical Trial Supply and Logistics Market Share Forecast by End Users, 2020, 2025, 2030 (%)
Figure 38. CRO’s Revenue Forecast 2020-2030 (US$ billion, AGR%)
Figure 39. CRO’s Market Forecast by Region 2020-2030 (US$ billion, AGR %)
Figure 40. Global CRO’s Market Share Forecast by Region 2020, 2025, 2030 (%)
Figure 41. CRO’s Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 42. CRO’s Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 43. CRO’s Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 44. CRO’s Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 45. Pharma & Biotech Revenue Forecast 2020-2030 (US$ billion, AGR%)
Figure 46. Pharma & Biotech Market Forecast by Region 2020-2030 (US$ billion, AGR %)
Figure 47. Global Pharma & Biotech Market Share Forecast by Region 2020, 2025, 2030 (%)
Figure 48. Pharma & Biotech Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 49. Pharma & Biotech Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 50. Pharma & Biotech Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 51. Others Revenue Forecast 2020-2030 (US$ billion, AGR%)
Figure 52. Other End Users Market Forecast by Region 2020-2030 (US$ billion, AGR %)
Figure 53. Global Other End Users Market Share Forecast by Region 2020, 2025, 2030 (%)
Figure 54. Others Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 55. Others Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 56. Others Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 57. Global Clinical Trial Supply and Logistics Market Forecast by Region 2020-2030 (US$ billion, AGR %)
Figure 58. Global Clinical Trial Supply and Logistics Market Share Forecast by Region 2020, 2025, 2030 (%)
Figure 59. North America Clinical Trial Supply and Logistics Market Forecast by Country 2020-2030 (US$ billion, AGR %)
Figure 60. North America Clinical Trial Supply and Logistics Market Share Forecast by Country 2020, 2025, 2030 (%)
Figure 61. North America Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR%)
Figure 62. North America Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 63. North America Clinical Trial Manufacturing Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 64. North America Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 65. North America Clinical Trial Logistics & Distribution Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 66. North America Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030 (US$ billion, AGR%)
Figure 67. U.S. Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 68. U.S. Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 69. U.S. Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 70. U.S. Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 71. U.S. Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 72. Canada Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 73. Canada Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 74. Canada Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 75. Canada Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 76. Canada Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 77. Europe Clinical Trial Supply and Logistics Market Forecast by Country 2020-2030 (US$ billion, AGR %)
Figure 78. Europe Clinical Trial Supply and Logistics Market Share Forecast by Country 2020, 2025, 2030 (%)
Figure 79. Europe Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR%)
Figure 80. Europe Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 81. Europe Clinical Trial Manufacturing Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 82. Europe Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 83. Europe Clinical Trial Logistics & Distribution Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 84. Europe Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030 (US$ billion, AGR%)
Figure 85. Germany Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR%)
Figure 86. Germany Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 87. Germany Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 88. Germany Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 89. Germany Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 90. France Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 91. France Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 92. France Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 93. France Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 94. France Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 95. UK Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 96. UK Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 97. UK Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 98. UK Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 99. UK Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 100. Italy Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 101. Italy Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 102. Italy Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 103. Italy Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 104. Italy Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 105. Spain Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 106. Spain Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 107. Spain Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 108. Spain Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 109. Spain Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 110. Rest of Europe Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 111. Rest of Europe Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 112. Rest of Europe Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 113. Rest of Europe Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 114. Rest of Europe Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 115. Asia Pacific Clinical Trial Supply and Logistics Market Forecast by Country 2020-2030 (US$ billion, AGR %)
Figure 116. Asia Pacific Clinical Trial Supply and Logistics Market Share Forecast by Country 2020, 2025, 2030 (%)
Figure 117. Asia Pacific Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR%)
Figure 118. Asia Pacific Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 119. Asia Pacific Clinical Trial Manufacturing Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 120. Asia Pacific Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 121. Asia Pacific Clinical Trial Logistics & Distribution Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 122. Asia Pacific Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030 (US$ billion, AGR%)
Figure 123. Japan Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 124. Japan Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 125. Japan Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 126. Japan Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 127. Japan Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 128. China Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 129. China Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 130. China Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 131. China Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 132. China Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 133. India Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 134. India Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 135. India Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 136. India Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 137. India Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 138. ASEAN Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 139. ASEAN Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 140. ASEAN Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 141. ASEAN Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 142. ASEAN Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 143. Rest of Asia Pacific Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 144. Rest of APAC Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 145. Rest of APAC Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 146. Rest of APAC Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 147. Rest of APAC Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 148. Latin America Clinical Trial Supply and Logistics Market Forecast by Country 2020-2030 (US$ billion, AGR %)
Figure 149. Latin America Clinical Trial Supply and Logistics Market Share Forecast by Country 2020, 2025, 2030 (%)
Figure 150. Latin America Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR%)
Figure 151. Latin America Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 152. Latin America Clinical Trial Manufacturing Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 153. Latin America Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 154. Latin America Clinical Trial Logistics & Distribution Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 155. Latin America Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030 (US$ billion, AGR%)
Figure 156. Brazil Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 157. Brazil Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 158. Brazil Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 159. Brazil Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 160. Brazil Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 161. Mexico Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 162. Mexico Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 163. Mexico Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 164. Mexico Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 165. Mexico Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 166. Rest of Latin America Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 167. Rest of LATAM Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 168. Rest of LATAM Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 169. Rest of LATAM Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 170. Rest of LATAM Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 171. Middle East and Africa Clinical Trial Supply and Logistics Market Forecast by Country 2020-2030 (US$ billion, AGR %)
Figure 172. Middle East and Africa Clinical Trial Supply and Logistics Market Share Forecast by Country 2020, 2025, 2030 (%)
Figure 173. Middle East & Africa Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR%)
Figure 174. MEA Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 175. MEA Clinical Trial Manufacturing Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 176. MEA Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %)
Figure 177. MEA Clinical Trial Logistics & Distribution Market Share Forecast by Type, 2020, 2025, 2030 (%)
Figure 178. Middle East & Africa Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030 (US$ billion, AGR%)
Figure 179. GCC Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 180. GCC Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 181. GCC Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 182. GCC Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 183. GCC Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 184. South Africa Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 185. South Africa Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 186. South Africa Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 187. South Africa Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 188. South Africa Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 189. Rest of Middle East and Africa Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %)
Figure 190. Rest of MEA Revenue Forecast 2020-2030 (US$ billion, AGR%): “V” Shaped Recovery
Figure 191. Rest of MEA Revenue Forecast 2020-2030 (US$ billion, AGR%): “U” Shaped Recovery
Figure 192. Rest of MEA Revenue Forecast 2020-2030 (US$ billion, AGR%): “W” Shaped Recovery
Figure 193. Rest of MEA Revenue Forecast 2020-2030 (US$ billion, AGR%): “L” Shaped Recovery
Figure 194. AmerisourceBergen Corporation Total Company Sales 2016-2019 (US$ million, AGR %)
Figure 195. DHL Total Company Sales 2016-2019 (€ million, AGR %)
Figure 196. MARKEN Total Company Sales 2016-2019 (US$ million, AGR %)
Figure 197. Fisher Clinical Services, Inc. Total Company Sales 2016-2019 (US$ million, AGR %)
Figure 198. PRA Health Sciences Total Company Sales 2016-2019 (US$ million, AGR %)
Figure 199. Catalent, Inc. Total Company Sales 2016-2019 (US$ million, AGR %)
Figure 200. FedEX Total Company Sales 2016-2019 (US$ million, AGR %)
Figure 201. Players in the Clinical Trial Supply Chain, 2020
Figure 202. Global Clinical Trial Supply and Logistics Market Forecast by Region 2020-2030 (US$ billion, AGR %)
Figure 203. Global Clinical Trial Supply and Logistics Market Share Forecast by Region 2020, 2025, 2030 (%)
List of Tables
Table 1. Global Clinical Trial Supply and Logistics Market by Region, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 2. OECD Economic Outlook Forecast, March 2020 (Percent change in Real GDP Growth)
Table 3. ICH Manufacturing Guidelines, 2001-2012
Table 4. Global Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 5. Clinical Trial Manufacturing Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%)
Table 6. Global Clinical Trial Manufacturing Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 7. Clinical Trial Manufacturing Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 8. Clinical Trial Manufacturing Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 9. Clinical Trial Manufacturing Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 10. Clinical Trial Manufacturing Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 11. Clinical Trial Logistics and Distribution Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%)
Table 12. Global Clinical Trial Logistics & Distribution Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 13. Clinical Trial Logistics & Distribution Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 14. Clinical Trial Logistics & Distribution Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 15. Clinical Trial Logistics & Distribution Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 16. Clinical Trial Logistics & Distribution Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 17. Clinical Trial Supply Chain Management Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%)
Table 18. Clinical Trial Supply Chain Management Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 19. Clinical Trial Supply Chain Management Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 20. Clinical Trial Supply Chain Management Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 21. Clinical Trial Supply Chain Management Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 22. Global Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 23. CRO’s Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%)
Table 24. CRO’s Market by Region, CAGR Forecast; 2020-2025, 2025-2030, 2020-2030
Table 25. CRO’s Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 26. CRO’s Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 27. CRO’s Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 28. CRO’s Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 29. Pharma & Biotech Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%)
Table 30. Pharma & Biotech Market by Region, CAGR Forecast; 2020-2025, 2025-2030, 2020-2030
Table 31. Pharma & Biotech Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 32. Pharma & Biotech Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 33. Pharma & Biotech Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 34. Pharma & Biotech Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 35. Others Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%)
Table 36. Other End Users Market by Region, CAGR Forecast; 2020-2025, 2025-2030, 2020-2030
Table 37. Others Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 38. Others Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 39. Others Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 40. Others Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 41. Global Clinical Trial Supply and Logistics Market CAGR Forecast by Region 2020, 2025, 2030 (%)
Table 42. North America Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 43. North America Clinical Trial Manufacturing Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 44. North America Clinical Trial Logistics & Distribution Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 45. North America Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 46. U.S. Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 47. U.S. Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 48. U.S. Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 49. U.S. Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 50. U.S. Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 51. Canada Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 52. Canada Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 53. Canada Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 54. Canada Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 55. Canada Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 56. Europe Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 57. Europe Clinical Trial Manufacturing Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 58. Europe Clinical Trial Logistics & Distribution Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 59. Europe Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 60. Germany Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 61. Germany Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 62. Germany Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 63. Germany Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 64. Germany Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 65. France Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 66. France Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 67. France Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 68. France Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 69. France Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 70. UK Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 71. UK Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 72. UK Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 73. UK Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 74. UK Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 75. Italy Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 76. Italy Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 77. Italy Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 78. Italy Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 79. Italy Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 80. Spain Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 81. Spain Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 82. Spain Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 83. Spain Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 84. Spain Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 85. Rest of Europe Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 86. Rest of Europe Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 87. Rest of Europe Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 88. Rest of Europe Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 89. Rest of Europe Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 90. Asia Pacific Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 91. Asia Pacific Clinical Trial Manufacturing Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 92. Asia Pacific Clinical Trial Logistics & Distribution Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 93. Asia Pacific Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 94. Japan Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 95. Japan Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 96. Japan Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 97. Japan Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 98. Japan Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 99. China Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 100. China Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 101. China Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 102. China Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 103. China Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 104. India Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 105. India Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 106. India Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 107. India Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 108. India Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 109. ASEAN Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 110. ASEAN Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 111. ASEAN Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 112. ASEAN Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 113. ASEAN Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 114. Rest of Asia Pacific Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 115. Rest of APAC Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 116. Rest of APAC Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 117. Rest of APAC Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 118. Rest of APAC Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 119. Latin America Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 120. Latin America Clinical Trial Manufacturing Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 121. Latin America Clinical Trial Logistics & Distribution Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 122. Latin America Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 123. Brazil Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 124. Brazil Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 125. Brazil Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 126. Brazil Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 127. Brazil Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 128. Mexico Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 129. Mexico Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 130. Mexico Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 131. Mexico Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 132. Mexico Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 133. Rest of Latin America Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 134. Rest of LATAM Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 135. Rest of LATAM Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 136. Rest of LATAM Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 137. Rest of LATAM Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 138. Middle East and Africa Clinical Trial Supply and Logistics Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 139. MEA Clinical Trial Manufacturing Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 140. MEA Clinical Trial Logistics & Distribution Market Forecast by Type, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 141. Middle East and Africa Clinical Trial Supply and Logistics Market Forecast by End Users, 2020-2030 (US$ billion, AGR %, CAGR %)
Table 142. GCC Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 143. GCC Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 144. GCC Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 145. GCC Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 146. GCC Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 147. South Africa Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 148. South Africa Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 149. South Africa Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 150. South Africa Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 151. South Africa Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 152. Rest of Middle East and Africa Clinical Trial Supply and Logistics Market Forecast 2020-2030 (US$ billion, AGR %, CAGR %)
Table 153. Rest of MEA Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “V” Shaped Recovery
Table 154. Rest of MEA Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “U” Shaped Recovery
Table 155. Rest of MEA Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “W” Shaped Recovery
Table 156. Rest of MEA Revenue Forecast 2020-2030 (US$ billion, AGR%, CAGR%): “L” Shaped Recovery
Table 157. AmerisourceBergen Corporation Profile 2020 (CEO, Headquarter, Business Segment in the Market, Founded, No. of Employees, Company Type, Website)
Table 158. AmerisourceBergen Corporation: Total Company Sales 2016-2019 (US$ million, AGR %)
Table 159. AmerisourceBergen Corporation Clinical Trial Supply and Logistics: Products / Services (Product, Specification / Features)
Table 160. AmerisourceBergen Corporation: Recent Developments (Date, Development, Details)
Table 161. DHL Profile 2020 (CEO, Headquarter, Business Segment in the Market, Founded, No. of Employees, Company Type, Website)
Table 162. DHL: Total Company Sales 2016-2019 (€ million, AGR %)
Table 163. DHL Clinical Trial Supply and Logistics: Products / Services (Product, Specification / Features)
Table 164. DHL: Recent Developments (Date, Development, Details)
Table 165. MARKEN Profile 2020 (CEO, Headquarter, Business Segment in the Market, Founded, No. of Employees, Company Type, Website)
Table 166. MARKEN Total Company Sales 2016-2019 (US$ million, AGR %)
Table 167. MARKEN Clinical Trials Supply and Logistics Products / Services (Product, Specification / Features)
Table 168. MARKEN Mergers and Acquisitions (Date, Development, Details)
Table 169. Fisher Clinical Services, Inc. Profile 2020 (CEO, Headquarter, Business Segment in the Market, Founded, No. of Employees, Company Type, Website)
Table 170. Fisher Clinical Services, Inc. Total Company Sales 2016-2019 (US$ million, AGR %)
Table 171. Fisher Clinical Services, Inc. Clinical Trials Supply and Logistics Products / Services (Product, Specification / Features)
Table 172. Ancillare, LP Profile 2020 (CEO, Total Company Sales US$ million, Headquarter, Business Segment in the Market, Founded, No. of Employees, Company Type, Website)
Table 173. Ancillare, LP Clinical Trials Supply and Logistics Products / Services (Product, Specification / Features)
Table 174. Ancillare, LP Mergers and Acquisitions (Date, Development, Details)
Table 175. Sharp Profile 2020 (CEO, Headquarter, Business Segment in the Market, Founded, No. of Employees, Company Type, Website)
Table 176. Sharp Clinical Trials Supply and Logistics Products / Services (Product, Specification / Features)
Table 177. Sharp Mergers and Acquisitions (Date, Development, Details)
Table 178. Endpoint Clinical, Inc. Profile 2020 (President, Headquarter, Business Segment in the Market, Founded, No. of Employees, Company Type, Website)
Table 179. Endpoint Clinical, Inc. Clinical Trials Supply and Logistics Products / Services (Product, Specification / Features)
Table 180. Endpoint Clinical, Inc. Mergers and Acquisitions (Date, Development, Details)
Table 181. PAREXEL International Corporation Profile 2020 (CEO, Headquarter, Business Segment in the Market, Founded, No. of Employees, Company Type, Website)
Table 182. PAREXEL International Corporation Total Company Sales 2016-2019 (US$ million, AGR %)
Table 183. PAREXEL International Corporation Clinical Trials Supply and Logistics Products / Services (Product, Specification / Features)
Table 184. PAREXEL International Corporation Mergers and Acquisitions (Date, Development, Details)
Table 185. PRA Health Sciences Profile 2020 (CEO, Total Company Sales US$ million, Headquarter, Business Segment in the Market, Founded, No. of Employees, Company Type, Website)
Table 186. PRA Health Sciences Total Company Sales 2016-2019 (US$ million, AGR %)
Table 187. PRA Health Sciences Clinical Trials Supply and Logistics Products / Services (Product, Specification / Features)
Table 188. PRA Health Sciences Mergers and Acquisitions (Date, Development, Details)
Table 189. Almac Group Profile 2020 (CEO, Headquarter, Business Segment in the Market, Founded, No. of Employees, Company Type, Website)
Table 190. Almac Group Clinical Trials Supply and Logistics Products / Services (Product, Specification / Features)
Table 191. Almac Group Mergers and Acquisitions (Date, Development, Details)
Table 192. Catalent, Inc. Profile 2020 (CEO, Headquarter, Business Segment in the Market, Founded, No. of Employees, Company Type, Website)
Table 193. Catalent, Inc. Total Company Sales 2016-2019 (US$ million, AGR %)
Table 194. Catalent, Inc. Clinical Trials Supply and Logistics Products / Services (Product, Specification / Features)
Table 195. Catalent, Inc. Mergers and Acquisitions (Date, Development, Details)
Table 196. The Coghlan Group: Clinical Trials Supply and Logistics Products / Services (Product, Specification / Features)
Table 197. The Coghlan Group Mergers and Acquisitions (Date, Development, Details)
Table 198. FedEX Profile 2020 (CEO, Headquarter, Business Segment in the Market, Founded, No. of Employees, Company Type, Website)
Table 199. FedEX: Total Company Sales 2016-2019 (US$ million, AGR %)
Table 200. FedEX: Clinical Trials Supply and Logistics Products / Services (Product, Specification / Features)
Table 201. FedEX Mergers and Acquisitions (Date, Development, Details)
Table 202. Benefits of Advanced Forecasting Tools for Clinical Trial Logistics, 2020
Table 203. Selected IVRS/IWRS Tools Available for Clinical Trials, 2020
Table 204. Home-Based Clinical Trial Distribution: Advantages and Disadvantages, 2020
Table 205. Outsourcing Clinical Trial Supply and Logistics: Advantages and Disadvantages, 2020
Table 206. Global Clinical Trial Supply and Logistics Market CAGR Forecast by Region 2020, 2025, 2030 (%)
Almac Group
AmerisourceBergen Corporation
Ancillare, LP
Catalent, Inc
DHL
Endpoint Clinical, Inc
FedEX
Fisher Clinical Services, Inc
MARKEN
Parexel International Corporation
PRA Health Sciences
Sharp
The Coghlan Group
List of Organizations Mentioned in the Report
Agência Nacional de Vigilância Sanitária (ANVISA)
Central Drugs Standard Control Organization (CDSCO)
China FDA (CFDA)
Chinese Ministry of Health
Comissão Nacional de Ética em Pesquisa (CONEP)
Consorcio de Apoyo a la Investigación Biomédica – Plataforma Española de Ensayos Clínicos (CAIBER)
Drug Controller General of India (DCGI)
European Commission
European Medicines Agency (EMA)
Food and Drug Administration (FDA)
Health Science Authority
International Air Transport Association (IATA)
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)
International Pharmaceutical Federation
Japanese Ministry of Health, Labour and Welfare (MHLW)
Les Enterprises du Medicament (LEEM)
Medicines and Healthcare Products Regulatory Agency (MHRA)
National Institutes of Health (NIH)
Pharmaceuticals for Human Use (ICH)
Transportation Administration Security (TAS)
US Pharmacopeia (USP)
World Health Organization (WHO)
Download sample pages
Complete the form below to download your free sample pages for Clinical Trial Supply and Logistics Market for Pharma 2020-2030
Related reports
-

Nasal Drug Delivery Technology Market Forecast 2020-2030
The Global Nasal Drug Delivery Technology Market is estimated at $44,385 m in 2019 and is expected to grow at...
Full DetailsPublished: 30 March 2020 -

Global Pharma Contract Sales Market Forecast 2020-2030
The global pharma contract sales market is expected to grow at a CAGR of 7.9% in the first half of...
Full DetailsPublished: 26 February 2020 -

Global Clinical Trial Management System Market Forecast 2020-2030
The global market for clinical trial management systems was valued to be $1,152m in 2019 and is valued to reach...
Full DetailsPublished: 17 March 2020 -

Pharmacokinetics Services Market Report 2020-2030
The rise in awareness regarding the importance of implementing pharmacokinetic studies during drug discovery and development have motivated the regulatory...
Full DetailsPublished: 01 January 1970 -

Clinical Microbiology Market Report 2020-2030
The growth of the global clinical microbiology market is owed to the rising geriatric population coupled with the growing incidence...
Full DetailsPublished: 01 January 1970 -

eClinical Solution Market Report 2019-2029
E-Clinical solution is an electronic medium of clinical study and research in pharmaceutical and biotechnical industry, the eClinical software helps...
Full DetailsPublished: 01 January 1970 -

Clinical Laboratory Tests Market Report 2021-2031
The global clinical laboratory tests market is intensely competitive market. Companies mostly contend with three types of suppliers of scientific...Full DetailsPublished: 18 February 2021 -

Vaccine Contract Manufacturing Market Forecast 2020-2030
The global vaccine contract manufacturing market was worth $2,241.6m in 2019 and is expected to grow at a CAGR of...
Full DetailsPublished: 08 April 2020 -

Active Pharmaceutical Ingredient Market Report 2020-2030
Where is the Active Pharmaceutical Ingredient market heading? If you are involved in this sector you must read this newly...
Full DetailsPublished: 01 January 1970 -

Patient Engagement Solutions Market Report 2020-2030
Visiongain’s report shows you the potential revenues streams to 2030, assessing data, trends, opportunities and business prospects there.
...Full DetailsPublished: 01 January 1970
Download sample pages
Complete the form below to download your free sample pages for Clinical Trial Supply and Logistics Market for Pharma 2020-2030
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Don’t Miss Out!
Subscribe to receive the latest Industry news, trending insight and analysis straight to your inbox.Choose your preferences:
Latest Pharma news
Retinal Gene Therapy Market
The global Retinal Gene Therapy market is projected to grow at a CAGR of 9.6% by 2034
26 July 2024
HIV Drugs and Injectables Market
The global HIV Drugs & Injectables market is projected to grow at a CAGR of 4.6 % by 2034
24 July 2024
Digital Twin Technology in Pharmaceutical Manufacturing Market
The global Digital Twin Technology in Pharmaceutical Manufacturing market is projected to grow at a CAGR of 31.3% by 2034
23 July 2024
Specialty Pharma Market
The global Specialty Pharma market is projected to grow at a CAGR of 7.5% by 2034
22 July 2024

















