Industries > Pharma > Biosimilars and Follow-On Biologics Market Report 2021-2031
Biosimilars and Follow-On Biologics Market Report 2021-2031
Forecasts by Type of Manufacturing (In-House, CMOs), by Type (Monoclonal Antibodies, Fusion Proteins, Insulin, Erythropoietin, Granulocyte-Colony Stimulating Factor, Interferon, Growth Hormones, Fertility Hormones, Others), by Application (Blood Disorders, Oncology Diseases, Chronic & Autoimmune Diseases, Growth Hormone Deficiencies, Others), by Technology (rDNA Technology, mAb Technology, Bioassay Technology) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Recovery Scenarios
Digital Transformations as a Key Enabler to Strengthen Research in Biosimilars Industry
The potential for digital & analytics applications in the pharmaceutical and biotechnology sector has grown dramatically in recent years as a result of fast technological advances as well as changes in the market environment & stakeholder behaviour as a result of the pandemic. Digital transformation has the potential to provide substantial advantages to the biosimilars industry across the value chain.
On the front end, technology has already played an important role in keeping the industry, health care providers, and pharmacists connected in the face of limited physical contacts. Traditional, face-to-face detailing-based commercial methods are increasingly and are being supplemented with digital engagement models, and this trend is expected to continue. This will not only allow the industry to deepen connections and thus increase biosimilar medicine adoption, but it will also provide an economically viable option for rapidly expanding reach into territories that would otherwise remain untapped due to constraints of the representative-based economic model.
At the backend, digital and analytics have the ability to significantly increase capacity by improving efficiency, quality results, and creating a zero-deviation environment. Through the use of simulations and in-silico batch modelling, drug development and product transfers processes would pick up speed in future.
Growing Prevalence of Lifestyle Diseases Anticipated to Fuel European Biosimilars Market Growth
Since biosimilars do not need significant research and testing, they are less costly than their branded equivalents. This saves both money and time, thus leading to reduction in overall price of the respective biosimilar. They also have short marketing periods since biosimilars do not need significant marketing because their branded equivalents’ safety and effectiveness profiles have previously been established. During the forecast period, many blockbuster biologics are anticipated to lose their patent protection. Biosimilar producers are anticipated to benefit greatly from the expiry of patents and other intellectual property rights. The European population is ageing, with approximately one-fifth of the population above 65 years which represents a substantial rise in the burden of lifestyle illnesses. Moreover, the incidence of illnesses such as diabetes, autoimmune disorders, cancer, and others are on rise. Due to increasing healthcare costs, governments in a number of European nations have enacted laws encouraging doctors, pharmacists, and patients to choose biosimilars over branded biologics.
Biosimilar drugs provide a significant potential with significant advantages, since they save money throughout Europe, contribute to the long-term viability of national healthcare systems, and enhance patient access to cutting-edge therapies. However, to realize these benefits over the course of long run, the market for biosimilar medicines must remain viable.
The global biosimilars and follow-on biologics is segmented on the basis of type of manufacturing into in-house, and CMOs segment. In-house segment dominated the global biosimilars market by manufacturing with a share of over 70% in 2020 .The in-house segment is projected to witness highest growth owing robust R&D infrastructure of major companies which closely work with their strategic partners.
The global biosimilars and follow-on biologics is segmented on the basis of type into monoclonal antibodies, fusion proteins, insulin, erythropoietin, granulocyte-colony stimulating factor, interferon, growth hormones, fertility hormones, and others. Monoclonal antibodies segment holds majority of the market share and was valued at US$8,910.0 million in 2020 growing at a CAGR of 30.84% from 2021 to 2031.
On the basis of application, the global biosimilars and follow-on biologics is segmented blood disorders, oncology diseases, chronic & autoimmune diseases, growth hormone deficiencies, and others. Oncology diseases segment held majority of the market share and was valued at US$3,704.8 million in 2020. The segment is expected to the highest growth rate during the forecast period.
According to Visiongain analysis, European region is a leader in the global biosimilars and follow-on biologics market. European biosimilars and follow-on biologics market was valued at US$5,308.0 million in 2020. This regional market growth has been fuelled by well-developed healthcare infrastructure and an increasing number of product releases.
What are the Market Trends for Global Biosimilars and Follow-on Biologics R&D Market?
• Regulatory Hurdles Slowing Down Access to Biosimilars
• Vulnerability of Global Pharmaceutical Manufacturing Amid COVID-19
• Emerging Economies Continues to Pose Challenges for Building Presence for Generics and Biosimilars Companies
• Digital Transformations as a Key Enabler to Strengthen Research in Biosimilars Industry
Discover how to stay ahead
Our 570+ page report provides 700+ tables and charts/graphs. Read on to discover the most lucrative areas in the industry and the future market prospects. Our new study lets you assess forecasted sales at overall world market and regional level. See financial results, trends, opportunities, and revenue predictions. Much opportunity remains in this growing Biosimilars and Follow-on Biologics R&D Market. See how to exploit the opportunities.
Forecasts to 2031 and other analyses reveal the commercial prospects
• In addition to revenue forecasting to 2031, our new study provides you with recent results, growth rates, and market shares.
• You find original analyses, with business outlooks and developments.
• Discover qualitative analyses (including market dynamics, drivers, opportunities, restraints, and challenges), PEST Analysis, Porter’s Five Forces, SWOT Analysis, product profiles and commercial developments.
Discover sales predictions for the world market and sub-markets
By Type of Manufacturing
• In-House
• Contract
By Type
• Monoclonal Antibodies
• Fusion Proteins
• Insulin
• Erythropoietin
• Granulocyte-Colony Stimulating Factor
• Interferon
• Growth Hormones
• Fertility Hormones
• Others
By Application
• Blood Disorders
• Oncology Diseases
• Chronic & Autoimmune Diseases
• Growth Hormone Deficiencies
• Others
By Technology
• RDNA Technology
• Mab Technology
• Bioassay Technology
In addition to the revenue predictions for the overall world market and segments, you will also find revenue forecasts for 5 regional and 14 leading national markets:
By Region
• North America
– U.S.
– Canada
• Europe
– Germany
– France
– UK
– Italy
– Spain
– Rest of Europe
• Asia Pacific
– China
– Japan
– India
– Rest of Asia Pacific
• Latin America
– Brazil
– Mexico
– Rest of Latin America
• Middle East & Africa
– GCC
– South Africa
– Rest of Middle East & Africa
Need industry data? Please contact us today.
Leading companies and the potential for market growth
Overall world revenue for Biosimilars and Follow-on Biologics R&D Market will surpass $xx billion in 2021, our work calculates. We predict strong revenue growth through to 2031. Our work identifies which organizations hold the greatest potential. Discover their capabilities, progress, and commercial prospects, helping you stay ahead.
How the Biosimilars and Follow-on Biologics R&D Market report helps you
In summary, our 570+ page report provides you with the following knowledge:
• Revenue forecasts to 2031 for Biosimilars and Follow-on Biologics R&D Market, with forecasts for Type of Manufacturing, Type, Application, and Technology, each forecasted at a global and regional level– discover the industry’s prospects, finding the most lucrative places for investments and revenues
• Revenue forecasts to 2031 for 5 regional and 14 key national markets– See forecasts for the Biosimilars and Follow-on Biologics R&D market in North America, Europe, Asia Pacific, and Rest of the World. These regional markets have been further bifurcated by countries including US, Canada, Brazil, Mexico, Germany, France, UK, Italy, Spain, China, India, Japan, Australia, South Korea, among other prominent economies.
• Prospects for established firms and those seeking to enter the market– including company profiles for 20 of the companies involved in the biosimilars and follow-on biologics R&D Market. Some of the company’s profiled in this report include 3SBio, Inc., AMEGA Biotech, Amgen Inc., Apotex, Inc., BIOCAD, Biocon Limited, Biogen, Inc., Celltrion Healthcare Co., Ltd., Coherus BioSciences, Dr. Reddy’s Laboratories Ltd., Eli Lilly and Company, Gedeon Richter PLC, Intas Pharmaceutical Ltd., Mabxience SA, Viatris Inc. (Mylan NV), Novartis AG, Pfizer Inc., Samsung Bioepis Co. Ltd., Stada Arzneimittel AG, and Teva Pharmaceutical among other prominent players.
Find quantitative and qualitative analyses with independent predictions. Receive information that only our report contains, staying informed with this invaluable business intelligence.
Information found nowhere else
With our newly report title, you are less likely to fall behind in knowledge or miss out on opportunities. See how our work could benefit your research, analyses, and decisions. Visiongain’s study is for everybody needing commercial analyses for the Biosimilars and Follow-on Biologics R&D Market and leading companies. You will find data, trends, and predictions.
To access the data contained in this document please email contactus@visiongain.com
Buy our report today Biosimilars and Follow-On Biologics Market Report 2021-2031: Forecasts by Type of Manufacturing (In-House, CMOs), by Type (Monoclonal Antibodies, Fusion Proteins, Insulin, Erythropoietin, Granulocyte-Colony Stimulating Factor, Interferon, Growth Hormones, Fertility Hormones, Others), by Application (Blood Disorders, Oncology Diseases, Chronic & Autoimmune Diseases, Growth Hormone Deficiencies, Others), by Technology (rDNA Technology, mAb Technology, Bioassay Technology) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Recovery Scenarios. Avoid missing out by staying informed – order our report now.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for a specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: sara.peerun@visiongain.com
1.1 Introduction to Biosimilars and Follow-on Biologics R&D Market
1.2 Biosimilars and Follow-on Biologics Market Definition
1.3 Why You Should Read This Report
1.4 What This Report Delivers
1.5 Key Questions Answered By This Analytical Report Include:
1.6 Who is This Report For?
1.7 Methodology
1.7.1 COVID-19 Impact: Recovery Scenarios
1.7.2 Market Evaluation & Forecasting Methodology
1.8 Frequently Asked Questions (FAQs)
1.9 Associated Visiongain Reports
1.10 About Visiongain
2 Executive Summary
2.1 Europe Leading the Worldwide Biosimilars Market
2.1.1 Emerging Biopharmaceutical Companies to Drive Biosimilars Market Growth Further Over the Next Decade
2.1.2 The EU as a Gateway to Global Expansion
2.1.3 Growing Demand to Strategize Healthcare Costs to Offer Lucrative Growth Opportunities for Market Players
3 Market Dynamics
3.1 Drivers
3.1.1 Incentivizing Providers to Adopt Biosimilars
3.1.2 Developed Regions Driving the Worldwide Biosimilars and Follow-on Biologics Market Growth
3.1.3 Biologics Patent Expiry Set to Open Fresh Revenue Pockets for Biosimilars Companies Over the Next Decade
3.1.4 Pricing Advantage of Biosimilars Comparison to Biologics Anticipated to Fuel Market Growth Through 2031
3.2 Restraints
3.2.1 High Cost Involvement, Uncertainty and Complexities in Manufacturing
3.2.2 Resistance from Biologics Manufacturers to Restrain Market Growth
3.2.3 Reluctance of Healthcare Providers to Switch to Biosimilars
3.3 Opportunities
3.3.1 Companies Can Use Information of Original Innovator thus Shorten Development & Approval Processes
3.3.2 Diversity in Approach Among the Authorities
3.4 Challenges
3.4.1 Lack of Incentive Strategies Across Geographies Anticipated to Challenge Market Growth
3.4.2 Building Trust Among End-Users is a Challenge for Market Growth
3.5 Trends
3.5.1 Regulatory Hurdles Slowing Down Access to Biosimilars
3.5.2 Vulnerability of Global Pharmaceutical Manufacturing Amid COVID-19
3.5.3 Emerging Economies Continues to Pose Challenges for Building Presence for Generics and Biosimilars Companies
3.5.4 Digital Transformations as a Key Enabler to Strengthen Research in Biosimilars Industry
3.6 Porter’s Five Forces Analysis
3.6.1 Buyer Power
3.6.2 Supplier Power
3.6.3 Competitive Rivalry
3.6.4 Threat of Substitute
3.6.5 Threat of New Entrants
3.7 PEST Analysis
3.7.1 Political Factors Impacting Biosimilars and Follow-on Biologics Market
3.7.2 Economic Factors Impacting Biosimilars and Follow-on Biologics Market
3.7.3 Social Factors Impacting Biosimilars and Follow-on Biologics Market
3.7.4 Technological Factors Impacting Biosimilars and Follow-on Biologics Market
4 Biosimilars and Follow-on Biologics Market by Type of Manufacturing
4.1 Introduction
4.2 In-House Segment Market Forecast, 2021-2031
4.2.1 COVID-19 Impact Recovery Scenarios (V, U, W, L)
4.3 CMOs Segment Market Forecast, 2021-2031
4.3.1 COVID-19 Impact Recovery Scenarios (V, U, W, L)
5 Biosimilars and Follow-on Biologics Market by Type
5.1 Introduction
5.2 Monoclonal Antibodies Segment Market Forecast, 2021-2031
5.2.1 Monoclonal Antibodies Segment Market Overview
5.2.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
5.3 Fusion Proteins Segment Market Forecast, 2021-2031
5.3.1 Fusion Protein Segment Market Overview
5.3.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
5.4 Insulin Segment Market Forecast, 2021-2031
5.4.1 Insulin Segment Market Overview
5.4.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
5.5 Erythropoietin Segment Market Forecast, 2021-2031
5.5.1 Erythropoietin Segment Market Overview
5.5.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
5.6 Granulocyte-Colony Stimulating Factor Segment Market Forecast, 2021-2031
5.6.1 G-CSF Segment Market Overview
5.6.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
5.7 Interferon Segment Market Forecast, 2021-2031
5.7.1 Interferon Segment Market Overview
5.7.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
5.8 Growth Hormones Segment Market Forecast, 2021-2031
5.8.1 Growth Hormones Segment Market Overview
5.8.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
5.9 Fertility Hormones Segment Market Forecast, 2021-2031
5.9.1 Fertility Hormones Segment Market Overview
5.9.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
5.10 Others Segment Market Forecast, 2021-2031
5.10.1 COVID-19 Impact Recovery Scenarios (V, U, W, L)
6 Biosimilars and Follow-on Biologics Market by Application
6.1 Blood Disorders Segment Market Forecast, 2021-2031
6.1.1 Blood Disorders Segment Market Overview
6.1.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
6.2 Oncology Diseases Segment Market Forecast, 2021-2031
6.2.1 Oncology Diseases Segment Market Overview
6.2.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
6.3 Chronic & Autoimmune Diseases Segment Market Forecast, 2021-2031
6.3.1 Chronic & Autoimmune Diseases Segment Market Overview
6.3.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
6.4 Growth Hormone Deficiencies Segment Market Forecast, 2021-2031
6.4.1 Growth Hormone Deficiencies Segment Market Overview
6.4.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
6.5 Others Segment Market Forecast, 2021-2031
6.5.1 COVID-19 Impact Recovery Scenarios (V, U, W, L)
7 Biosimilars and Follow-on Biologics Market by Technology
7.1 rDNA Technology Segment Market Forecast, 2021-2031
7.1.1 rDNA Technology Segment Market Overview
7.1.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
7.2 mAb Technology Segment Market Forecast, 2021-2031
7.2.1 mAb Technology Segment Market Overview
7.2.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
7.3 Bioassay Technology Segment Market Forecast, 2021-2031
7.3.1 Bioassay Technology Segment Market Overview
7.3.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
8 Regional and Leading National Biosimilars and Follow-on Biologics Market Forecasts 2021-2031
8.1 Global Biosimilars and Follow-on Biologics Market by Region Forecast 2021-2031
8.2 COVID-19 Impact Recovery Scenarios (V, U, W, L)
9 North America Biosimilars and Follow-on Biologics Market
9.1 North America Biosimilars and Follow-on Biologics Market by Country, Forecast 2021-2031
9.2 North America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing
9.3 North America Biosimilars and Follow-on Biologics Market Forecast by Type
9.4 North America Biosimilars and Follow-on Biologics Market Forecast by Application
9.5 North America Biosimilars and Follow-on Biologics Market Forecast by Technology
9.6 U.S. Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
9.6.1 United States is Just Beginning to Catch Up European Biosimilars Market
9.6.2 Rising Biosimilar Uptake to Fuel U.S. Biosimilars Market Growth
9.6.3 Advancements in Biosimilars Development and Approvals Across the United States to Fuel Market Growth
9.6.4 COVID-19 Impact Recovery Scenarios (V, U, W, L): U.S. Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
9.7 Canada Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
9.7.1 Biosimilar Authorization Activity Across Canadian Region
9.7.2 Initiatives by Health Canada to Boost Canadian Biosimilars and Follow-on Biologics Market Over the Next Decade
9.7.3 Availability of Biosimilars in Oncology Treatment Landscape Driving Regional Growth
9.7.4 Canada Biosimilars and Follow-on Biologics Market Outlook
9.7.5 COVID-19 Impact Recovery Scenarios (V, U, W, L): Canada Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
10 Europe Biosimilars and Follow-on Biologics Market
10.1 Growing Prevalence of Lifestyle Diseases Anticipated to Fuel European Biosimilars Market Growth
10.2 Continued Benefits of Biosimilars to Offer Lucrative Growth Prospects in Long Term
10.3 Europe Biosimilars and Follow-on Biologics Market by Country, Forecast 2021-2031
10.4 Europe Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing
10.5 Europe Biosimilars and Follow-on Biologics Market Forecast by Type
10.6 Europe Biosimilars and Follow-on Biologics Market Forecast by Application
10.7 Europe Biosimilars and Follow-on Biologics Market Forecast by Technology
10.8 Germany Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
10.8.1 Biosimilars Have Been Accepted as an Important Component of Proper Medication Usage in Germany
10.8.2 Germany Intends to Switch to Biologics Pharmacies by August 2022 thus Jeopardising the Long-Term Viability of Biosimilars
10.8.3 Germany Biosimilars and Follow-on Biologics Market Outlook
10.8.4 COVID-19 Impact Recovery Scenarios (V, U, W, L): Germany Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
10.9 UK Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
10.9.1 NHS Encouraging the Growth of Biosimilar Manufacturing in United Kingdom?
10.9.2 Despite Price Reduction, Biosimilar Medications are not Widely Used in Retail Settings?
10.9.3 UK Biosimilars and Follow-on Biologics Market Outlook
10.9.4 COVID-19 Impact Recovery Scenarios (V, U, W, L): UK Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
10.10 France Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
10.10.1 How is the Biosimilar Pricing Situation Across France?
10.10.2 Initiatives taken by French Government to Offer Lucrative Growth Prospects for Biosimilars Manufacturers Over the Forecast Period
10.10.3 COVID-19 Impact Recovery Scenarios (V, U, W, L): France Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
10.11 Italy Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
10.11.1 Italian Government Policies Anticipated to Fuel Development of Biosimilars Market Across the Region
10.11.2 Biosimilars to Help Reduce Price of Pharmaceuticals Across the Italian Region
10.11.3 COVID-19 Impact Recovery Scenarios (V, U, W, L): Italy Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
10.12 Spain Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
10.12.1 Spain Biosimilars and Follow-on Biologics Market Outlook
10.12.2 Budget Impact Analysis of Biosimilar Products in Spain
10.12.3 COVID-19 Impact Recovery Scenarios (V, U, W, L): Spain Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
10.13 Rest of Europe Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
10.13.1 Rest of Europe Biosimilars and Follow-on Biologics Market Outlook
10.13.2 COVID-19 Impact Recovery Scenarios (V, U, W, L): Rest of Europe Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
11 Asia Pacific Biosimilars and Follow-on Biologics Market
11.1 Low Clinical Development Costs Coupled with Regulatory Framework Ease to Fuel Regional Growth
11.2 Indian Pharmaceutical Companies Have an Enormous Scope in the Biosimilars Market
11.3 Asia Pacific Biosimilars and Follow-on Biologics Market by Country, Forecast 2021-2031
11.4 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing
11.5 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Type
11.6 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Application
11.7 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Technology
11.8 Japan Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
11.8.1 Biosimilars to Bolster Japanese Healthcare System Growth Over the Next Decade
11.8.2 Japan still has a slow growth in this sector compared to US and EU
11.8.3 Introduction of Biosimilars to Boost Regional Growth Through 2031
11.8.4 Ageing Japanese Population Demanding the Development of Biosimilars Over the Forecast Period
11.8.5 COVID-19 Impact Recovery Scenarios (V, U, W, L): Japan Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
11.9 China Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
11.9.1 Significant Surge in Biosimilar Approvals Over The Past 3 Years to Drive Market Growth
11.9.2 Technological Advancements to Fuel Chinese Biosimilars Market Growth Over the Forecast Period
11.9.3 Expansion by Major Firms to Boost Chinese Biosimilars Market Growth Through 2031
11.9.4 Overcrowding of Chinese Market Has Led to the Withdrawal of International Players
11.9.5 COVID-19 Impact Recovery Scenarios (V, U, W, L): China Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
11.10 India Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
11.10.1 India Set to Become Sixth Largest Pharmaceutical Market By End of 2030
11.10.2 Renowned Pharmaceutical Companies Establishing Partnerships with Indian Pharma Companies
11.10.3 India Biosimilars and Follow-on Biologics Market Outlook
11.10.4 COVID-19 Impact Recovery Scenarios (V, U, W, L): India Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
11.11 Rest of Asia Pacific Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
11.11.1 South Korean Biopharma Companies Jostling to Capture Significant Share of Asia Pacific Biosimilars Market
11.11.2 COVID-19 Impact Recovery Scenarios (V, U, W, L): Rest of Asia Pacific Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
12 Latin America Biosimilars and Follow-on Biologics Market
12.1 Latin America Biosimilars and Follow-on Biologics Market by Country, Forecast 2021-2031
12.2 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing
12.3 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type
12.4 Latin America Biosimilars and Follow-on Biologics Market Forecast by Application
12.5 Latin America Biosimilars and Follow-on Biologics Market Forecast by Technology
12.6 Brazil Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
12.6.1 Brazilian Biosimilars Regulations
12.6.2 Brazil Biosimilars and Follow-on Biologics Market Outlook
12.6.3 COVID-19 Impact Recovery Scenarios (V, U, W, L): Brazil Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
12.7 Mexico Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
12.7.1 Mexican Biosimilars Market to Offer Lucrative Growth Prospects Across Latin American Region
12.7.2 Mexico Biosimilars and Follow-on Biologics Market Outlook
12.7.3 COVID-19 Impact Recovery Scenarios (V, U, W, L): Mexico Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
12.8 Rest of Latin America Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
12.8.1 COVID-19 Impact Recovery Scenarios (V, U, W, L): Rest of Latin America Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
13 Middle East and Africa Biosimilars and Follow-on Biologics Market
13.1 Middle East and Africa Biosimilars and Follow-on Biologics Market by Country, Forecast 2021-2031
13.2 MEA Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing
13.3 MEA Biosimilars and Follow-on Biologics Market Forecast by Type
13.4 MEA Biosimilars and Follow-on Biologics Market Forecast by Application
13.5 MEA Biosimilars and Follow-on Biologics Market Forecast by Technology
13.6 GCC Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
13.6.1 GCC Gearing Up to Take Big Jump on Biosimilars
13.6.2 Collaborations Among Pharmaceutical Firms Across GCC to Offer Lucrative Growth Prospects for Market Players Over the Forecast Period
13.6.3 COVID-19 Impact Recovery Scenarios (V, U, W, L): GCC Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
13.7 South Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
13.7.1 South African Evolving Demographic and Epidemiological Profile to Offer Lucrative Growth Prospects
13.7.2 Presence of Major Pharmaceutical Firms to Intensify Competition
13.7.3 COVID-19 Impact Recovery Scenarios (V, U, W, L): South Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
13.8 Rest of Middle East and Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
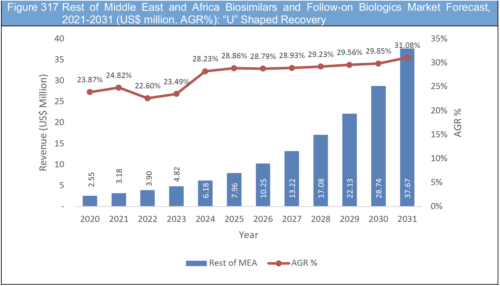
13.8.1 COVID-19 Impact Recovery Scenarios (V, U, W, L): Rest of Middle East and Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031
14 Company Profiles
14.1 Biosimilars Company Ranking, 2020
14.2 3SBio, Inc.
14.2.1 Company Snapshot
14.2.2 Company Overview
14.2.3 Product Benchmarking
14.2.4 Recent Developments
14.3 AMEGA Biotech
14.3.1 Company Snapshot
14.3.2 Company Overview
14.3.3 Product Benchmarking
14.4 Amgen Inc.
14.4.1 Company Snapshot
14.4.2 Company Overview
14.4.3 Financial Analysis
14.4.4 Product Benchmarking
14.4.5 Recent Developments
14.5 Apotex, Inc.
14.5.1 Company Snapshot
14.5.2 Company Overview
14.5.3 Product Benchmarking
14.6 BIOCAD
14.6.1 Company Snapshot
14.6.2 Company Overview
14.6.3 Product Benchmarking
14.6.4 Recent Developments
14.7 Biocon Limited
14.7.1 Company Snapshot
14.7.2 Company Overview
14.7.3 Financial Analysis
14.7.4 Product Benchmarking
14.7.5 Recent Developments
14.8 Biogen, Inc.
14.8.1 Company Snapshot
14.8.2 Company Overview
14.8.3 Product Benchmarking
14.9 Celltrion Healthcare Co.,Ltd.
14.9.1 Company Snapshot
14.9.2 Company Overview
14.9.3 Product Benchmarking
14.9.4 Recent Developments
14.10 Coherus BioSciences
14.10.1 Company Snapshot
14.10.2 Company Overview
14.10.3 Product Benchmarking
14.10.4 Recent Developments
14.11 Dr. Reddy’s Laboratories Ltd.
14.11.1 Company Snapshot
14.11.2 Company Overview
14.11.3 Product Benchmarking
14.12 Eli Lilly and Company
14.12.1 Company Snapshot
14.12.2 Company Overview
14.12.3 Financial Analysis
14.12.4 Product Benchmarking
14.12.5 Recent Developments
14.13 Gedeon Richter PLC
14.13.1 Company Snapshot
14.13.2 Company Overview
14.13.3 Product Benchmarking
14.13.4 Recent Developments
14.14 Intas Pharmaceutical Ltd.
14.14.1 Company Snapshot
14.14.2 Company Overview
14.14.3 Product Benchmarking
14.15 Mabxience SA
14.15.1 Company Snapshot
14.15.2 Company Overview
14.15.3 Product Benchmarking
14.15.4 Recent Developments
14.16 Viatris Inc. (Mylan NV)
14.16.1 Company Snapshot
14.16.2 Company Overview
14.16.3 Financial Analysis
14.16.4 Product Benchmarking
14.16.5 Recent Developments
14.17 Novartis AG
14.17.1 Company Snapshot
14.17.2 Company Overview
14.17.3 Financial Analysis
14.17.4 Product Benchmarking
14.17.5 Recent Developments
14.18 Pfizer Inc.
14.18.1 Company Snapshot
14.18.2 Company Overview
14.18.3 Financial Analysis
14.18.4 Product Benchmarking
14.18.5 Recent Developments
14.19 Samsung Bioepis Co. Ltd.
14.19.1 Company Snapshot
14.19.2 Company Overview
14.19.3 Product Benchmarking
14.19.4 Recent Developments
14.20 Stada Arzneimittel AG
14.20.1 Company Snapshot
14.20.2 Company Overview
14.20.3 Financial Analysis
14.20.4 Product Benchmarking
14.20.5 Recent Developments
14.21 Teva Pharmaceutical
14.21.1 Company Snapshot
14.21.2 Company Overview
14.21.3 Financial Analysis
14.21.4 Product Benchmarking
14.21.5 Recent Developments
15 Conclusion and Recommendations
15.1 Concluding Remarks
15.2 Recommendations
15.3 Europe Leading the Global Biosimilars Market
List of Tables
Table 1 Global Biosimilars and Follow-on Biologics Market Snapshot, 2021 & 2031 (US$ million, CAGR %)
Table 2 Global Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 3 Global Biosimilars and Follow-on Biologics Market Forecast for In-House Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 4 Global Biosimilars and Follow-on Biologics Market Forecast for In-House Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 5 Global Biosimilars and Follow-on Biologics Market Forecast for In-House Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 6 Global Biosimilars and Follow-on Biologics Market Forecast for In-House Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 7 Global Biosimilars and Follow-on Biologics Market Forecast for In-House Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 8 Global Biosimilars and Follow-on Biologics Market Forecast for CMOs Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 9 Global Biosimilars and Follow-on Biologics Market Forecast for CMOs Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 10 Global Biosimilars and Follow-on Biologics Market Forecast for CMOs Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 11 Global Biosimilars and Follow-on Biologics Market Forecast for CMOs Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 12 Global Biosimilars and Follow-on Biologics Market Forecast for CMOs Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 13 Global Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 14 Global Biosimilars and Follow-on Biologics Market Forecast for Monoclonal Antibodies Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 15 Global Biosimilars and Follow-on Biologics Market Forecast for Monoclonal Antibodies Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 16 Global Biosimilars and Follow-on Biologics Market Forecast for Monoclonal Antibodies Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 17 Global Biosimilars and Follow-on Biologics Market Forecast for Monoclonal Antibodies Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 18 Global Biosimilars and Follow-on Biologics Market Forecast for Monoclonal Antibodies Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 19 Global Biosimilars and Follow-on Biologics Market Forecast for Fusion Proteins Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 20 Global Biosimilars and Follow-on Biologics Market Forecast for Fusion Proteins Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 21 Global Biosimilars and Follow-on Biologics Market Forecast for Fusion Proteins Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 22 Global Biosimilars and Follow-on Biologics Market Forecast for Fusion Proteins Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 23 Global Biosimilars and Follow-on Biologics Market Forecast for Fusion Proteins Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 24 Global Biosimilars and Follow-on Biologics Market Forecast for Insulin Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 25 Global Biosimilars and Follow-on Biologics Market Forecast for Insulin Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 26 Global Biosimilars and Follow-on Biologics Market Forecast for Insulin Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 27 Global Biosimilars and Follow-on Biologics Market Forecast for Insulin Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 28 Global Biosimilars and Follow-on Biologics Market Forecast for Insulin Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 29 Global Biosimilars and Follow-on Biologics Market Forecast for Erythropoietin Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 30 Global Biosimilars and Follow-on Biologics Market Forecast for Erythropoietin Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 31 Global Biosimilars and Follow-on Biologics Market Forecast for Erythropoietin Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 32 Global Biosimilars and Follow-on Biologics Market Forecast for Erythropoietin Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 33 Global Biosimilars and Follow-on Biologics Market Forecast for Erythropoietin Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 34 Global Biosimilars and Follow-on Biologics Market Forecast for Granulocyte-Colony Stimulating Factor Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 35 Global Biosimilars and Follow-on Biologics Market Forecast for Granulocyte-Colony Stimulating Factor Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 36 Global Biosimilars and Follow-on Biologics Market Forecast for Granulocyte-Colony Stimulating Factor Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 37 Global Biosimilars and Follow-on Biologics Market Forecast for Granulocyte-Colony Stimulating Factor Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 38 Global Biosimilars and Follow-on Biologics Market Forecast for Granulocyte-Colony Stimulating Factor Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 39 Global Biosimilars and Follow-on Biologics Market Forecast for Interferon Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 40 Global Biosimilars and Follow-on Biologics Market Forecast for Interferon Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 41 Global Biosimilars and Follow-on Biologics Market Forecast for Interferon Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 42 Global Biosimilars and Follow-on Biologics Market Forecast for Interferon Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 43 Global Biosimilars and Follow-on Biologics Market Forecast for Interferon Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 44 Global Biosimilars and Follow-on Biologics Market Forecast for Growth Hormones Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 45 Global Biosimilars and Follow-on Biologics Market Forecast for Growth Hormones Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 46 Global Biosimilars and Follow-on Biologics Market Forecast for Growth Hormones Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 47 Global Biosimilars and Follow-on Biologics Market Forecast for Growth Hormones Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 48 Global Biosimilars and Follow-on Biologics Market Forecast for Growth Hormones Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 49 Global Biosimilars and Follow-on Biologics Market Forecast for Fertility Hormones Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 50 Global Biosimilars and Follow-on Biologics Market Forecast for Fertility Hormones Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 51 Global Biosimilars and Follow-on Biologics Market Forecast for Fertility Hormones Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 52 Global Biosimilars and Follow-on Biologics Market Forecast for Fertility Hormones Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 53 Global Biosimilars and Follow-on Biologics Market Forecast for Fertility Hormones Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 54 Global Biosimilars and Follow-on Biologics Market Forecast for Others Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 55 Global Biosimilars and Follow-on Biologics Market Forecast for Others Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 56 Global Biosimilars and Follow-on Biologics Market Forecast for Others Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 57 Global Biosimilars and Follow-on Biologics Market Forecast for Others Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 58 Global Biosimilars and Follow-on Biologics Market Forecast for Others Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 59 Global Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 60 Global Biosimilars and Follow-on Biologics Market Forecast for Blood Disorders Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 61 Global Biosimilars and Follow-on Biologics Market Forecast for Blood Disorders Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 62 Global Biosimilars and Follow-on Biologics Market Forecast for Blood Disorders Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 63 Global Biosimilars and Follow-on Biologics Market Forecast for Blood Disorders Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 64 Global Biosimilars and Follow-on Biologics Market Forecast for Blood Disorders Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 65 Global Biosimilars and Follow-on Biologics Market Forecast for Oncology Diseases Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 66 Global Biosimilars and Follow-on Biologics Market Forecast for Oncology Diseases Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 67 Global Biosimilars and Follow-on Biologics Market Forecast for Oncology Diseases Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 68 Global Biosimilars and Follow-on Biologics Market Forecast for Oncology Diseases Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 69 Global Biosimilars and Follow-on Biologics Market Forecast for Oncology Diseases Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 70 Global Biosimilars and Follow-on Biologics Market Forecast for Chronic & Autoimmune Diseases Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 71 Global Biosimilars and Follow-on Biologics Market Forecast for Chronic & Autoimmune Diseases Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 72 Global Biosimilars and Follow-on Biologics Market Forecast for Chronic & Autoimmune Diseases Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 73 Global Biosimilars and Follow-on Biologics Market Forecast for Chronic & Autoimmune Diseases Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 74 Global Biosimilars and Follow-on Biologics Market Forecast for Chronic & Autoimmune Diseases Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 75 Global Biosimilars and Follow-on Biologics Market Forecast for Growth Hormone Deficiencies Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 76 Global Biosimilars and Follow-on Biologics Market Forecast for Growth Hormone Deficiencies Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 77 Global Biosimilars and Follow-on Biologics Market Forecast for Growth Hormone Deficiencies Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 78 Global Biosimilars and Follow-on Biologics Market Forecast for Growth Hormone Deficiencies Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 79 Global Biosimilars and Follow-on Biologics Market Forecast for Growth Hormone Deficiencies Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 80 Global Biosimilars and Follow-on Biologics Market Forecast for Others Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 81 Global Biosimilars and Follow-on Biologics Market Forecast for Others Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 82 Global Biosimilars and Follow-on Biologics Market Forecast for Others Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 83 Global Biosimilars and Follow-on Biologics Market Forecast for Others Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 84 Global Biosimilars and Follow-on Biologics Market Forecast for Others Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 85 Global Biosimilars and Follow-on Biologics Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 86 Global Biosimilars and Follow-on Biologics Market Forecast for rDNA Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 87 Global Biosimilars and Follow-on Biologics Market Forecast for rDNA Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 88 Global Biosimilars and Follow-on Biologics Market Forecast for rDNA Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 89 Global Biosimilars and Follow-on Biologics Market Forecast for rDNA Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 90 Global Biosimilars and Follow-on Biologics Market Forecast for rDNA Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 91 Global Biosimilars and Follow-on Biologics Market Forecast for Mab Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 92 Global Biosimilars and Follow-on Biologics Market Forecast for Mab Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 93 Global Biosimilars and Follow-on Biologics Market Forecast for Mab Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 94 Global Biosimilars and Follow-on Biologics Market Forecast for Mab Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 95 Global Biosimilars and Follow-on Biologics Market Forecast for Mab Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 96 Global Biosimilars and Follow-on Biologics Market Forecast for Bioassay Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 97 Global Biosimilars and Follow-on Biologics Market Forecast for Bioassay Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 98 Global Biosimilars and Follow-on Biologics Market Forecast for Bioassay Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 99 Global Biosimilars and Follow-on Biologics Market Forecast for Bioassay Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 100 Global Biosimilars and Follow-on Biologics Market Forecast for Bioassay Technology Segment, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 101 Global Biosimilars and Follow-on Biologics Market Forecast by Region, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 102 Global Biosimilars and Follow-on Biologics Market Forecast by Region, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 103 Global Biosimilars and Follow-on Biologics Market Forecast by Region, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 104 Global Biosimilars and Follow-on Biologics Market Forecast by Region, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 105 Global Biosimilars and Follow-on Biologics Market Forecast by Region, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 106 North America Biosimilars and Follow-on Biologics Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 107 North America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 108 North America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 109 North America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 110 North America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 111 North America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 112 North America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 113 North America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 114 North America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 115 North America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 116 North America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 117 North America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 118 North America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 119 North America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 120 North America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 121 North America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 122 North America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 123 North America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 124 North America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 125 North America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 126 North America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 127 Biosimilars Launched in the United States as of December 2020
Table 128 Biosimilars Approved in the United States
Table 129 U.S. Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 130 U.S. Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 131 U.S. Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 132 U.S. Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 133 U.S. Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 134 Canada Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 135 Canada Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 136 Canada Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 137 Canada Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 138 Canada Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 139 Europe Biosimilars and Follow-on Biologics Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 140 Europe Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 141 Europe Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 142 Europe Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 143 Europe Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 144 Europe Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 145 Europe Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 146 Europe Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 147 Europe Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 148 Europe Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 149 Europe Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 150 Europe Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 151 Europe Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 152 Europe Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 153 Europe Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 154 Europe Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 155 Europe Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 156 Europe Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 157 Europe Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 158 Europe Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 159 Europe Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 160 Germany Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 161 Germany Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 162 Germany Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 163 Germany Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 164 Germany Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 165 UK Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 166 UK Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 167 UK Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 168 UK Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 169 UK Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 170 France Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 171 France Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 172 France Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 173 France Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 174 France Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 175 Italy Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 176 Italy Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 177 Italy Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 178 Italy Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 179 Italy Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 180 Spain Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 181 Spain Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 182 Spain Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 183 Spain Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 184 Spain Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 185 Rest of Europe Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 186 Rest of Europe Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 187 Rest of Europe Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 188 Rest of Europe Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 189 APAC Biosimilars and Follow-on Biologics Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 190 APAC Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 191 APAC Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 192 APAC Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 193 APAC Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 194 APAC Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 195 APAC Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 196 APAC Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 197 APAC Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 198 APAC Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 199 APAC Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 200 APAC Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 201 APAC Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 202 APAC Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 203 APAC Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 204 APAC Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 205 APAC Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 206 APAC Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 207 APAC Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 208 APAC Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 209 APAC Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 210 Japan Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 211 Japan Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 212 Japan Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 213 Japan Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 214 Japan Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 215 China Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 216 China Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 217 China Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 218 China Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 219 China Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 220 India Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 221 India Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 222 India Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 223 India Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 224 India Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 225 Rest of Asia Pacific Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 226 Rest of Asia Pacific Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 227 Rest of Asia Pacific Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 228 Rest of Asia Pacific Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 229 Rest of Asia Pacific Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 230 LATAM Biosimilars and Follow-on Biologics Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 231 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 232 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 233 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 234 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 235 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 236 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 237 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 238 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 239 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 240 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 241 Latin America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 242 Latin America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 243 Latin America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 244 Latin America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 245 Latin America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 246 Latin America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 247 Latin America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 248 Latin America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 249 Latin America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 250 Latin America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 251 Brazil Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 252 Brazil Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 253 Brazil Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 254 Brazil Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 255 Brazil Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 256 Mexico Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 257 Mexico Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 258 Mexico Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 259 Mexico Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 260 Mexico Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 261 Rest of Latin America Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 262 Rest of Latin America Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 263 Rest of Latin America Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 264 Rest of Latin America Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 265 MEA Biosimilars and Follow-on Biologics Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 266 MEA Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 267 MEA Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 268 MEA Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 269 MEA Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 270 MEA Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 271 MEA Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 272 MEA Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 273 MEA Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 274 MEA Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 275 MEA Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 276 MEA Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 277 MEA Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 278 MEA Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 279 MEA Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 280 MEA Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 281 MEA Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 282 MEA Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 283 MEA Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 284 MEA Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 285 GCC Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 286 GCC Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 287 GCC Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 288 GCC Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 289 GCC Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 290 South Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 291 South Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 292 South Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 293 South Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 294 South Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 295 Rest of MEA Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 296 Rest of MEA Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 297 Rest of MEA Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 298 Rest of MEA Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 299 Rest of MEA Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 300 Global Biosimilars Market: Top 5 Companies, 2020
Table 301 3SBio, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 302 3SBio, Inc.: Product Benchmarking
Table 303 3SBio, Inc.: Recent Developments
Table 304 AMEGA Biotech: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 305 AMEGA Biotech: Product Benchmarking
Table 306 Amgen Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 307 Amgen Inc.: Product Benchmarking
Table 308 Amgen Inc.: Recent Developments
Table 309 Apotex, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 310 Apotex, Inc.: Product Benchmarking
Table 311 BIOCAD: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 312 BIOCAD: Product Benchmarking
Table 313 BIOCAD: Recent Developments
Table 314 Biocon Limited: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 315 Biocon Limited: Product Benchmarking
Table 316 Biocon Limited: Recent Developments
Table 317 Biogen, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 318 Biogen, Inc.: Product Benchmarking
Table 319 Celltrion Healthcare Co.,Ltd.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 320 Celltrion Healthcare Co.,Ltd.: Product Benchmarking
Table 321 Celltrion Healthcare Co.,Ltd.: Recent Developments
Table 322 Coherus BioSciences: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 323 Coherus BioSciences: Product Benchmarking
Table 324 Coherus BioSciences: Recent Developments
Table 325 Dr. Reddy’s Laboratories Ltd.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 326 Dr. Reddy’s Laboratories Ltd.: Product Benchmarking
Table 327 Eli Lilly and Company: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 328 Eli Lilly and Company: Product Benchmarking
Table 329 Eli Lilly and Company: Recent Developments
Table 330 Gedeon Richter PLC: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 331 Gedeon Richter PLC: Product Benchmarking
Table 332 Gedeon Richter PLC: Recent Developments
Table 333 Intas Pharmaceutical Ltd.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 334 Intas Pharmaceutical Ltd.: Product Benchmarking
Table 335 Mabxience SA: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 336 Mabxience SA: Product Benchmarking
Table 337 Mabxience SA: Recent Developments
Table 338 Viatris Inc. (Mylan NV): Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 339 Viatris Inc. (Mylan NV): Product Benchmarking
Table 340 Viatris Inc. (Mylan NV): Recent Developments
Table 341 Novartis AG: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 342 Novartis AG: Product Benchmarking
Table 343 Novartis AG: Recent Developments
Table 344 Pfizer Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 345 Pfizer Inc.: Product Benchmarking
Table 346 Pfizer Inc.: Recent Developments
Table 347 Samsung Bioepis Co. Ltd.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 348 Samsung Bioepis Co. Ltd.: Product Benchmarking
Table 349 Samsung Bioepis Co. Ltd.: Recent Developments
Table 350 Stada Arzneimittel AG: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 351 Stada Arzneimittel AG: Product Benchmarking
Table 352 Stada Arzneimittel AG: Recent Developments
Table 353 Teva Pharmaceutical: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 354 Teva Pharmaceutical: Recent Developments
List of Figures
Figure 1 Global Biosimilars and Follow-on Biologics Market: Market Segmentation
Figure 2 Global Biosimilars and Follow-on Biologics Leading Regions Revenue: 2021, 2026 & 2031; CAGR % (2021-2031)
Figure 3 Global Biosimilars and Follow-on Biologics Market: Market Attractiveness Index
Figure 4 Global Biosimilars and Follow-on Biologics Market Dynamics
Figure 5 Biosimilars Approval in U.S. & Europe, 2015-2020
Figure 6 Biologics Patent Expiry, 2020-2030
Figure 7 Porter’s Analysis
Figure 8 PEST Analysis
Figure 9 Global Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR %)
Figure 10 Global Biosimilars and Follow-on Biologics Market Share Forecast by Type of Manufacturing, 2021, 2026, 2031 (%)
Figure 11 Global Biosimilars and Follow-on Biologics Market for In-House Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 12 Global Biosimilars and Follow-on Biologics Market for In-House Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 13 Global Biosimilars and Follow-on Biologics Market for In-House Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 14 Global Biosimilars and Follow-on Biologics Market for In-House Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 15 Global Biosimilars and Follow-on Biologics Market for In-House Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 16 Global Biosimilars and Follow-on Biologics Market for CMOs Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 17 Global Biosimilars and Follow-on Biologics Market for CMOs Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 18 Global Biosimilars and Follow-on Biologics Market for CMOs Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 19 Global Biosimilars and Follow-on Biologics Market for CMOs Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 20 Global Biosimilars and Follow-on Biologics Market for CMOs Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 21 Global Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR %)
Figure 22 Global Biosimilars and Follow-on Biologics Market Share Forecast by Type, 2021, 2026, 2031 (%)
Figure 23 Global Biosimilars and Follow-on Biologics Market for Monoclonal Antibodies Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 24 Global Biosimilars and Follow-on Biologics Market for Monoclonal Antibodies Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 25 Global Biosimilars and Follow-on Biologics Market for Monoclonal Antibodies Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 26 Global Biosimilars and Follow-on Biologics Market for Monoclonal Antibodies Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 27 Global Biosimilars and Follow-on Biologics Market for Monoclonal Antibodies Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 28 Global Biosimilars and Follow-on Biologics Market for Fusion Proteins Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 29 Global Biosimilars and Follow-on Biologics Market for Fusion Proteins Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 30 Global Biosimilars and Follow-on Biologics Market for Fusion Proteins Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 31 Global Biosimilars and Follow-on Biologics Market for Fusion Proteins Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 32 Global Biosimilars and Follow-on Biologics Market for Fusion Proteins Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 33 Global Biosimilars and Follow-on Biologics Market for Insulin Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 34 Global Biosimilars and Follow-on Biologics Market for Insulin Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 35 Global Biosimilars and Follow-on Biologics Market for Insulin Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 36 Global Biosimilars and Follow-on Biologics Market for Insulin Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 37 Global Biosimilars and Follow-on Biologics Market for Insulin Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 38 Global Biosimilars and Follow-on Biologics Market for Erythropoietin Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 39 Global Biosimilars and Follow-on Biologics Market for Erythropoietin Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 40 Global Biosimilars and Follow-on Biologics Market for Erythropoietin Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 41 Global Biosimilars and Follow-on Biologics Market for Erythropoietin Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 42 Global Biosimilars and Follow-on Biologics Market for Erythropoietin Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 43 Global Biosimilars and Follow-on Biologics Market for Granulocyte-Colony Stimulating Factor Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 44 Global Biosimilars and Follow-on Biologics Market for Granulocyte-Colony Stimulating Factor Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 45 Global Biosimilars and Follow-on Biologics Market for Granulocyte-Colony Stimulating Factor Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 46 Global Biosimilars and Follow-on Biologics Market for Granulocyte-Colony Stimulating Factor Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 47 Global Biosimilars and Follow-on Biologics Market for Granulocyte-Colony Stimulating Factor Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 48 Global Biosimilars and Follow-on Biologics Market for Interferon Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 49 Global Biosimilars and Follow-on Biologics Market for Interferon Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 50 Global Biosimilars and Follow-on Biologics Market for Interferon Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 51 Global Biosimilars and Follow-on Biologics Market for Interferon Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 52 Global Biosimilars and Follow-on Biologics Market for Interferon Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 53 Global Biosimilars and Follow-on Biologics Market for Growth Hormones Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 54 Global Biosimilars and Follow-on Biologics Market for Growth Hormones Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 55 Global Biosimilars and Follow-on Biologics Market for Growth Hormones Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 56 Global Biosimilars and Follow-on Biologics Market for Growth Hormones Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 57 Global Biosimilars and Follow-on Biologics Market for Growth Hormones Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 58 Global Biosimilars and Follow-on Biologics Market for Fertility Hormones Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 59 Global Biosimilars and Follow-on Biologics Market for Fertility Hormones Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 60 Global Biosimilars and Follow-on Biologics Market for Fertility Hormones Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 61 Global Biosimilars and Follow-on Biologics Market for Fertility Hormones Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 62 Global Biosimilars and Follow-on Biologics Market for Fertility Hormones Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 63 Global Biosimilars and Follow-on Biologics Market for Others Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 64 Global Biosimilars and Follow-on Biologics Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 65 Global Biosimilars and Follow-on Biologics Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 66 Global Biosimilars and Follow-on Biologics Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 67 Global Biosimilars and Follow-on Biologics Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 68 Global Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 69 Global Biosimilars and Follow-on Biologics Market Share Forecast by Application, 2021, 2026, 2031 (%)
Figure 70 Global Biosimilars and Follow-on Biologics Market for Blood Disorders Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 71 Global Biosimilars and Follow-on Biologics Market for Blood Disorders Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 72 Global Biosimilars and Follow-on Biologics Market for Blood Disorders Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 73 Global Biosimilars and Follow-on Biologics Market for Blood Disorders Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 74 Global Biosimilars and Follow-on Biologics Market for Blood Disorders Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 75 Global Biosimilars and Follow-on Biologics Market for Oncology Diseases Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 76 Global Biosimilars and Follow-on Biologics Market for Oncology Diseases Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 77 Global Biosimilars and Follow-on Biologics Market for Oncology Diseases Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 78 Global Biosimilars and Follow-on Biologics Market for Oncology Diseases Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 79 Global Biosimilars and Follow-on Biologics Market for Oncology Diseases Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 80 Global Biosimilars and Follow-on Biologics Market for Chronic & Autoimmune Diseases Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 81 Global Biosimilars and Follow-on Biologics Market for Chronic & Autoimmune Diseases Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 82 Global Biosimilars and Follow-on Biologics Market for Chronic & Autoimmune Diseases Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 83 Global Biosimilars and Follow-on Biologics Market for Chronic & Autoimmune Diseases Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 84 Global Biosimilars and Follow-on Biologics Market for Chronic & Autoimmune Diseases Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 85 Global Biosimilars and Follow-on Biologics Market for Growth Hormone Deficiencies Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 86 Global Biosimilars and Follow-on Biologics Market for Growth Hormone Deficiencies Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 87 Global Biosimilars and Follow-on Biologics Market for Growth Hormone Deficiencies Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 88 Global Biosimilars and Follow-on Biologics Market for Growth Hormone Deficiencies Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 89 Global Biosimilars and Follow-on Biologics Market for Growth Hormone Deficiencies Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 90 Global Biosimilars and Follow-on Biologics Market for Others Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 91 Global Biosimilars and Follow-on Biologics Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 92 Global Biosimilars and Follow-on Biologics Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 93 Global Biosimilars and Follow-on Biologics Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 94 Global Biosimilars and Follow-on Biologics Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 95 Global Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR %)
Figure 96 Global Biosimilars and Follow-on Biologics Market Share Forecast by Technology, 2021, 2026, 2031 (%)
Figure 97 Global Biosimilars and Follow-on Biologics Market for rDNA Technology Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 98 Global Biosimilars and Follow-on Biologics Market for rDNA Technology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 99 Global Biosimilars and Follow-on Biologics Market for rDNA Technology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 100 Global Biosimilars and Follow-on Biologics Market for rDNA Technology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 101 Global Biosimilars and Follow-on Biologics Market for rDNA Technology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 102 Global Biosimilars and Follow-on Biologics Market for Mab Technology Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 103 Global Biosimilars and Follow-on Biologics Market for Mab Technology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 104 Global Biosimilars and Follow-on Biologics Market for Mab Technology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 105 Global Biosimilars and Follow-on Biologics Market for Mab Technology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 106 Global Biosimilars and Follow-on Biologics Market for Mab Technology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 107 Global Biosimilars and Follow-on Biologics Market for Bioassay Technology Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 108 Global Biosimilars and Follow-on Biologics Market for Bioassay Technology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 109 Global Biosimilars and Follow-on Biologics Market for Bioassay Technology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 110 Global Biosimilars and Follow-on Biologics Market for Bioassay Technology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 111 Global Biosimilars and Follow-on Biologics Market for Bioassay Technology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 112 Global Biosimilars and Follow-on Biologics Market Forecast by Region 2021-2031 (US$ million)
Figure 113 Global Biosimilars and Follow-on Biologics Market Share Forecast by Region 2021, 2026, 2031 (%)
Figure 114 Global Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 115 Global Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 116 Global Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 117 Global Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 118 North America Biosimilars and Follow-on Biologics Market Forecast by Country 2021-2031 (US$ million)
Figure 119 North America Biosimilars and Follow-on Biologics Market Share Forecast by Country 2021, 2026, 2031 (%)
Figure 120 North America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%)
Figure 121 North America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 122 North America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 123 North America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 124 North America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 125 North America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%)
Figure 126 North America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 127 North America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 128 North America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 129 North America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 130 North America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%)
Figure 131 North America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 132 North America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 133 North America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 134 North America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 135 North America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%)
Figure 136 North America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 137 North America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 138 North America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 139 North America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 140 U.S. Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 141 U.S. Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 142 U.S. Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 143 U.S. Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 144 U.S. Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 145 Canada Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 146 Canada Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 147 Canada Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 148 Canada Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 149 Canada Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 150 Europe Biosimilars and Follow-on Biologics Market Forecast by Country 2021-2031 (US$ million)
Figure 151 Europe Biosimilars and Follow-on Biologics Market Share Forecast by Country 2021, 2026, 2031 (%)
Figure 152 Europe Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%)
Figure 153 Europe Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 154 Europe Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 155 Europe Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 156 Europe Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 157 Europe Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%)
Figure 158 Europe Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 159 Europe Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 160 Europe Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 161 Europe Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 162 Europe Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%)
Figure 163 Europe Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 164 Europe Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 165 Europe Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 166 Europe Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 167 Europe Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%)
Figure 168 Europe Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 169 Europe Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 170 Europe Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 171 Europe Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 172 Germany Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 173 Germany Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 174 Germany Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 175 Germany Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 176 Germany Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 177 UK Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 178 UK Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 179 UK Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 180 UK Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 181 UK Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 182 France Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 183 France Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 184 France Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 185 France Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 186 France Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 187 Italy Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 188 Italy Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 189 Italy Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 190 Italy Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 191 Italy Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 192 Spain Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 193 Spain Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 194 Spain Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 195 Spain Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 196 Spain Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 197 Rest of Europe Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 198 Rest of Europe Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 199 Rest of Europe Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 200 Rest of Europe Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 201 Rest of Europe Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 202 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Country 2021-2031 (US$ million)
Figure 203 Asia Pacific Biosimilars and Follow-on Biologics Market Share Forecast by Country 2021, 2026, 2031 (%)
Figure 204 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%)
Figure 205 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 206 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 207 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 208 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 209 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%)
Figure 210 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 211 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 212 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 213 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 214 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%)
Figure 215 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 216 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 217 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 218 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 219 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%)
Figure 220 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 221 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 222 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 223 Asia Pacific Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 224 Biosimilar Approvals in Japan, 2010-2020
Figure 225 Japan Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 226 Japan Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 227 Japan Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 228 Japan Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 229 Japan Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 230 Biosimilar Approvals in China v.s Japan & South Korea, 2015-2020
Figure 231 China Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 232 China Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 233 China Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 234 China Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 235 China Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 236 India Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 237 India Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 238 India Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 239 India Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 240 India Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 241 Rest of Asia Pacific Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 242 Rest of Asia Pacific Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 243 Rest of Asia Pacific Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 244 Rest of Asia Pacific Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 245 Rest of Asia Pacific Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 246 Latin America Biosimilars and Follow-on Biologics Market Forecast by Country 2021-2031 (US$ million)
Figure 247 Latin America Biosimilars and Follow-on Biologics Market Share Forecast by Country 2021, 2026, 2031 (%)
Figure 248 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%)
Figure 249 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 250 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 251 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 252 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 253 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%)
Figure 254 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 255 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 256 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 257 Latin America Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 258 Latin America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%)
Figure 259 Latin America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 260 Latin America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 261 Latin America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 262 Latin America Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 263 Latin America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%)
Figure 264 Latin America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 265 Latin America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 266 Latin America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 267 Latin America Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 268 Brazil Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 269 Brazil Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 270 Brazil Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 271 Brazil Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 272 Brazil Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 273 Mexico Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 274 Mexico Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 275 Mexico Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 276 Mexico Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 277 Mexico Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 278 Rest of Latin America Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 279 Rest of Latin America Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 280 Rest of Latin America Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 281 Rest of Latin America Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 282 Rest of Latin America Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 283 Middle East and Africa Biosimilars and Follow-on Biologics Market Forecast by Country 2021-2031 (US$ million)
Figure 284 Middle East and Africa Biosimilars and Follow-on Biologics Market Share Forecast by Country 2021, 2026, 2031 (%)
Figure 285 MEA Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%)
Figure 286 MEA Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 287 MEA Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 288 MEA Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 289 MEA Biosimilars and Follow-on Biologics Market Forecast by Type of Manufacturing, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 290 MEA Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%)
Figure 291 MEA Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 292 MEA Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 293 MEA Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 294 MEA Biosimilars and Follow-on Biologics Market Forecast by Type, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 295 MEA Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%)
Figure 296 MEA Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 297 MEA Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 298 MEA Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 299 MEA Biosimilars and Follow-on Biologics Market Forecast by Application, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 300 MEA Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%)
Figure 301 MEA Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 302 MEA Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 303 MEA Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 304 MEA Biosimilars and Follow-on Biologics Market Forecast by Technology, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 305 GCC Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 306 GCC Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 307 GCC Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 308 GCC Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 309 GCC Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 310 South Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 311 South Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 312 South Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 313 South Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 314 South Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 315 Rest of Middle East and Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 316 Rest of Middle East and Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 317 Rest of Middle East and Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 318 Rest of Middle East and Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 319 Rest of Middle East and Africa Biosimilars and Follow-on Biologics Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 320 Amgen Inc.: Net Revenue, 2015-2020 (US$ million, AGR%)
Figure 321 Amgen Inc.: R&D Expenses, 2015-2020 (US$ million, AGR%)
Figure 322 Amgen Inc.: Gross Profit, 2015-2020 (US$ million, AGR%)
Figure 323 Biocon Limited: Net Revenue, 2017-2020 (US$ million, AGR%)
Figure 324 Biocon Limited: R&D Expenses, 2017-2020 (US$ million, AGR%)
Figure 325 Biocon Limited: Biosimilars Sales, 2017-2020 (US$ million, AGR%)
Figure 326 Biocon Limited: Business Segment Market Share, 2020 (%)
Figure 327 Biocon Limited: Geographic Market Share, 2020 (%)
Figure 328 Eli Lilly and Company: Net Revenue, 2015-2020 (US$ million, AGR%)
Figure 329 Eli Lilly and Company: R&D Expenses, 2015-2020 (US$ million, AGR%)
Figure 330 Eli Lilly and Company: Gross Profit, 2015-2020 (US$ million, AGR%)
Figure 331 Eli Lilly and Company: Geographic Revenue, 2018-2020 (US$ million, AGR%)
Figure 332 Viatris Inc. (Mylan NV): Net Revenue, 2015-2020 (US$ million, AGR%)
Figure 333 Viatris Inc. (Mylan NV): R&D Expenses, 2015-2020 (US$ million, AGR%)
Figure 334 Viatris Inc. (Mylan NV): Gross Profit, 2015-2020 (US$ million, AGR%)
Figure 335 Novartis AG: Net Revenue, 2015-2020 (US$ million, AGR%)
Figure 336 Novartis AG: R&D Expenses, 2015-2020 (US$ million, AGR%)
Figure 337 Novartis AG: Gross Profit, 2015-2020 (US$ million, AGR%)
Figure 338 Novartis AG: Net Sales by Business Segment, 2019-2020 (US$ million)
Figure 339 Novartis AG: Net sales to third parties by business franchise in the Sandoz Division, 2020 (%)
Figure 340 Pfizer Inc.: Net Revenue, 2015-2020 (US$ million, AGR%)
Figure 341 Pfizer Inc.: R&D Expenses, 2015-2020 (US$ million, AGR%)
Figure 342 Pfizer Inc.: Gross Profit, 2015-2020 (US$ million, AGR%)
Figure 343 Pfizer Inc.: Geographic Revenue Share, 2018-2020 (%)
Figure 344 Stada Arzneimittel AG: Net Revenue, 2016-2020 (US$ million, AGR%)
Figure 345 Stada Arzneimittel AG: R&D Expenses, 2018-2020 (US$ million, AGR%)
Figure 346 Stada Arzneimittel AG: Gross Profit, 2016-2020 (US$ million, AGR%)
Figure 347 Stada Arzneimittel AG: Net Income, 2019-2020 (%)
Figure 348 Teva Pharmaceutical: Net Revenue, 2015-2020 (US$ million, AGR%)
Figure 349 Teva Pharmaceutical: R&D Expenses, 2015-2020 (US$ million, AGR%)
Figure 350 Teva Pharmaceutical: Gross Profit, 2015-2020 (US$ million, AGR%)
Figure 351 Global Biosimilars and Follow-on Biologics Market Share Forecast by Region 2021, 2026, 2031 (%)
3SBio, Inc.
AMEGA Biotech
Amgen Inc.
Apotex, Inc.
BIOCAD
Biocon Limited
Biogen, Inc.
Celltrion Healthcare Co. Ltd.
Coherus BioSciences
Dr. Reddy's Laboratories Ltd.
Eli Lilly and Company
Gedeon Richter PLC
Intas Pharmaceutical Ltd.
Mabxience SA
Novartis AG
Pfizer Inc.
Samsung Bioepis Co. Ltd.
Stada Arzneimittel AG
Teva Pharmaceutical
Viatris Inc. (Mylan NV)
List of Other Companies
Alteogen
Amega Biotech
Apotex
Astellas Pharma
BioXpress Therapeutics
Boehringer Ingelheim
Bristol-Myers Squibb
CCM Duopharma
Dong-A Pharmaceutical
Dr. Reddy’s Laboratories
Eisai
Eli Lilly
Emcure Pharmaceuticals
Eurofarma
FibroGen
Fuji Pharma
Gan & Lee
Gennova
Geropharm
Gilead Sciences
GlaxoSmithKline
Intas Biopharmaceuticals
JCR Pharmaceuticals
Qilu Pharmaceutical
Ranbaxy Laboratories
Regeneron Pharmaceuticals
Reliance Life Sciences (RLS)
Virchow Biotech
Wanbang Biopharmaceuticals
Wockhardt
Xiamen Amoytop Biotech
Zenotech
List of Organizations
Argentine Ministry of Health
Cardiovascular and Renal Drugs Advisory Committee
Cardiovascular Drugs Advisory Committee
Centers of Medicare and Medicaid Services (CMS)
Central Drugs Standard Control Organisation
Centre for Drug Evaluation (CDE)
China Food and Drug Administration
Comisión Federal para la Protección contra Riesgos Sanitarios
Committee for Medicinal Products for Human Use
European Generic Medicines Association (EGA)
European Medicines Agency (EMA)
Intellectual Property Appellate Board (IPAB)
Korea Food and Drug Administration
Mexican Supreme Court
Ministry for Health Labour and Welfare (MHLW)
National Health Service (NHS)
National Institute for Health and Care Excellence (NICE)
Norwegian Ministry of Health
Pan American Health Organization
Pharmaceuticals and Medical Devices Agency (PMDA)
Russian Ministry of Health
Scottish Medicines Consortium (SMC)
Stanford University
State Employees' Social Security and Social Services Institute (ISSSTE)
The Brazilian Ministry of Health
The Drug Regulatory Authority of Pakistan
The Ministry of Food and Drug Safety (MFDS)
The National Conference of State Legislatures (US)
The United Laboratories (TUL)
Therapeutic Goods Administration
Toronto University
UK Medicines and Healthcare Products Regulatory Agency (MHRA)
University of California, San Francisco
University of Tokyo
US Center for Disease Prevention and Control (CDC)
US Court of Appeals
US Food and Drug Administration (FDA)
US Patent and Trademark Office
World Health Organization (WHO)
Download sample pages
Complete the form below to download your free sample pages for Biosimilars and Follow-On Biologics Market Report 2021-2031
Related reports
-

Biopharmaceuticals Contract Manufacturing Market Report 2022-2032
Ageing population will be more susceptible to such diseases and is one of the key factors driving the growth of...Full DetailsPublished: 26 January 2022 -

Single-use Bioprocessing Probes & Sensors Market Report 2022-2032
To promote their organic revenue growth and market presence, the top companies are pursuing different strategic initiatives such as OEM...Full DetailsPublished: 05 January 2022 -

Biologics Market Report 2022-2032
The global biologics market was valued at US$382.85 billion in 2022 and is projected to grow at a CAGR of...Full DetailsPublished: 04 July 2022 -

Contract Manufacturing Outsourcing (CMO) of Sterile Injectable Drugs Market Report 2021-2031
Contract manufacturing outsourcing is gaining attraction owing to growing trend of outsourcing among pharmaceutical businesses. ...Full DetailsPublished: 16 November 2021 -

Gene Therapy R&D Market Report 2021-2031
Our new study lets you assess forecasted sales at overall world market and regional level. See financial results, trends, opportunities,...Full DetailsPublished: 11 June 2021 -

Clinical Trial Supplies Market Report 2021-2031
Deviating from protocols raises the danger of missing or delaying data collection from current investigations. This highlights the growing importance...Full DetailsPublished: 11 October 2021 -

Cell Therapy Technologies Market Report 2021-2031
Cell therapy technology is one of the fastest growing markets and is an ever-changing landscape. It represents the umbrella of...Full DetailsPublished: 10 March 2021 -

Pharmaceutical Spray Drying Market Report 2021-2031
The rising prevalence of oncological disorders, lung infections, rising demand for customised drugs coupled with new orphan diseases discoveries, are...Full DetailsPublished: 25 October 2021 -

Biological Drug API Manufacturing Services Market Report 2021-2031
Demand in the early years of the forecast period will come from multinational biotech looking to sign deals with Indian...Full DetailsPublished: 07 January 2021 -

Biosimilar Monoclonal Antibodies Market Report 2022-2032
The global biosimilar monoclonal antibodies market was valued at US$4,087 million in 2021 and is projected to grow at a...Full DetailsPublished: 25 July 2022
Download sample pages
Complete the form below to download your free sample pages for Biosimilars and Follow-On Biologics Market Report 2021-2031
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Latest Pharma news
Visiongain Publishes Drug Delivery Technologies Market Report 2024-2034
The global Drug Delivery Technologies market is estimated at US$1,729.6 billion in 2024 and is projected to grow at a CAGR of 5.5% during the forecast period 2024-2034.
23 April 2024
Visiongain Publishes Cell Therapy Technologies Market Report 2024-2034
The cell therapy technologies market is estimated at US$7,041.3 million in 2024 and is projected to grow at a CAGR of 10.7% during the forecast period 2024-2034.
18 April 2024
Visiongain Publishes Automation in Biopharma Industry Market Report 2024-2034
The global Automation in Biopharma Industry market is estimated at US$1,954.3 million in 2024 and is projected to grow at a CAGR of 7% during the forecast period 2024-2034.
17 April 2024
Visiongain Publishes Anti-obesity Drugs Market Report 2024-2034
The global Anti-obesity Drugs market is estimated at US$11,540.2 million in 2024 and is expected to register a CAGR of 21.2% from 2024 to 2034.
12 April 2024


















