Industries > Pharma > Viral Vectors & Plasmid DNA Manufacturing Market Report 2021-2031
Viral Vectors & Plasmid DNA Manufacturing Market Report 2021-2031
Forecasts by Vector Types (Adenovirus, Retrovirus, Plasmid DNA, AAV, Lentivirus, Others), Application (Antisense & RNAi, Gene Therapy, Cell Therapy, Vaccinology), Disease (Oncology, Genetic Disorders, Infectious Diseases, Others), End-use (Pharma and Biopharma Companies, Research Institutes), by Region (North America, Europe, Asia Pacific, Latin America, Middle East & Africa). PLUS Analysis of Leading Viral Vectors & Plasmid DNA Manufacturing Companies AND COVID-19 Recovery Scenarios
What are the Regulatory Challenges for Viral Vectors for Cell and Gene Therapy?
The processing of viral vectors is complex. It is difficult to scale up legacy technologies adopted from the academic research side, for one thing. Technology issues combined with talent shortages are also a further bottleneck to capacity expansion. The demand for relatively young cells and gene therapy is extremely fragmented worldwide. In order to assist with viral vector demands and shortages, few contract research and development organizations (CMO/CROs) are commercially scaled. Just a handful of larger corporations are capable of manufacturing on a commercial scale, and there are few solutions that enable businesses to increase their own capability.
What are the Technological Advancements in Manufacturing of Viral Vectors?
Gene therapies are incredibly costly in this early stage of growth, but they deliver the promise of being curative in a single phase, thus reducing overall healthcare costs. Many disorders may be treated by recent advances in the discovery of genes responsible for diseases and the use of gene editing technology for genetic modification. As a consequence, biopharmaceutical manufacturers are focused on the rapid market deployment of goods. Their initiatives include designing facilities and supply chains to meet the growing demands of patients worldwide, lowering production costs, the effectiveness and efficacy of these new modalities, and improving patient safety by minimizing the possible side effects of gene therapy drugs.
UNIQUE COVID-19 VARIATIONS– only available in this Visiongain report are dedicated analysis of 4 different rebound scenarios of how the market will develop – no matter how COVID-19 affects the economy.
To access the data contained in this document please email contactus@visiongain.com
Which Factors are Fueling Viral Vectors and Plasmid DNA Manufacturing Industry Growth?
– Robust Pipeline for Gene Therapy and Viral Vectors
– Increasing Capacities by Manufacturers Owing to Rising Demand
Which Factors are Restraining Growth?
– High Cost of Therapies
– Bottlenecks Experienced by Manufacturers
What are the Market Opportunities & Threats?
– Rise in the Development of Allogenic and Autologous Cell Therapy
– Lucrative opportunity in manufacturing of COVID-19 vaccine
– Increasing Applications as Preclinical Tool in Neuroscience Research
How do prominent players strengthen their position throughout the world?
You must read this newly updated report if you are involved in this sector. The report from Visiongain shows you potential revenues up to 2031, evaluate information, trends, opportunities and business outlooks.
Discover how to stay ahead
Our 580+ page report provides 500+ tables and charts/graphs. Read on to discover the most lucrative areas in the industry and the future market prospects. Our new study lets you assess forecasted sales at overall world market and regional level. See financial results, trends, opportunities, and revenue predictions. Much opportunity remains in this growing Viral Vectors and Plasmid DNA Manufacturing Market. See how to exploit the opportunities.
Forecasts to 2031 and other analyses reveal the commercial prospects
• In addition to revenue forecasting to 2031, our new study provides you with recent results, growth rates, and market shares.
• You find original analyses, with business outlooks and developments.
• Discover qualitative analyses (including market dynamics, drivers, opportunities, restraints and challenges), SWOT Analysis, Porter’s Analysis, product profiles and commercial developments.
Discover sales predictions for the world market and submarkets
Vector Type
• Adenovirus
• Retrovirus
• Plasmid DNA
• AAV
• Lentivirus
• Others
Application
• Antisense & RNAi
• Gene Therapy
• Cell Therapy
• Vaccinology
Disease
• Oncology
• Genetic Disorders
• Infectious Diseases
• Others
End-Use
• Pharma and Biopharma Companies
• Research Institutes
In addition to the revenue predictions for the overall world market and segments, you will also find revenue forecasts for 5 regional and 9 leading national markets:
By Region
• North America
– U.S.
– Canada
• Europe
– Germany
– UK
– Rest of Europe
• Asia Pacific
– China
– Japan
– India
– Rest of Asia Pacific
• Latin America
– Brazil
– Rest of Latin America
• Middle East and Africa
– South Africa
– Rest of MEA
Need industry data? Please contact us today.
Leading companies and the potential for market growth
Overall world revenue for Viral Vectors and Plasmid DNA Manufacturing Market will surpass $xx million in 2020, our work calculates. We predict strong revenue growth through to 2031. Our work identifies which regions hold the greatest potential. Discover their capabilities, progress, and commercial prospects, helping you stay ahead.
Prospects for established firms and those seeking to enter the market– including company profiles for 32 of the major companies involved in the Viral Vectors and Plasmid DNA Manufacturing Market. Some of the companies profiled in this report include Merck KGaA, Lonza, FUJIFILM Diosynth Biotechnologies U.S.A., Inc., Cobra Biologics Ltd., Brammer Bio, Waisman Biomanufacturing, Genezen, YPOSKESI, Advanced BioScience Laboratories, Inc. (ABL, Inc.), Novasep Holding S.A.S, ATVIO Biotech Ltd, Vigene Biosciences, Inc., Cytiva, CEVEC Pharmaceuticals GmbH, Batavia Biosciences B.V, Biovian Oy, Wuxi AppTec Co., Ltd., VGXI, Inc., Paragon Bioservices, Inc., Miltenyi Biotec GmbH, SIRION Biotech GmbH, Virovek Incorporation, BioNTech IMFS GmbH, VIVEbiotech S.L., Creative Biogene, Vibalogics GmbH, Cell and Gene Therapy Catapult, BlueBird Bio, Addgene, Inc., Aldevron, L.L.C., Audentes Therapeutics, and BioMarin Pharmaceutical.
Key Questions Answered by this Report:
– What is the current size of the overall global viral vectors and plasmid DNA manufacturing market? How much will this market be worth from 2021 to 2031?
– What are the main drivers and restraints that will shape the overall viral vectors and plasmid DNA manufacturing market over the next ten years?
– What are the main segments within the overall viral vectors and plasmid DNA manufacturing market? How much will each of these segments be worth for the period 2021 to 2031? How will the composition of the market change during that time, and why?
– What factors will affect that industry and market over the next ten years?
– What are the largest national markets for the world viral vectors and plasmid DNA manufacturing? What is their current status and how will they develop over the next ten years? What are their revenue potentials to 2031?
– How will market shares of the leading national markets change by 2031, and which geographical region will lead the market in 2031?
– Which are the leading companies and what are their activities, results, developments, and prospects?
– What are the main trends that will affect the world Viral Vectors and Plasmid DNA Manufacturing market between 2021 and 2031?
– What are the main strengths, weaknesses, opportunities, and threats for the market?
– How will the global viral vectors and plasmid DNA manufacturing market evolve over the forecasted period, 2021 to 2031?
– How will market shares of prominent national markets change from 2021, and which countries will lead the market in 2031, achieving highest revenues and fastest growth?
Find quantitative and qualitative analyses with independent predictions. Receive information that only our report contains, staying informed with this invaluable business intelligence.
Information found nowhere else
With our newly report title, you are less likely to fall behind in knowledge or miss out on opportunities. See how our work could benefit your research, analyses, and decisions.
Visiongain’s study is intended for everybody needing commercial analyses for the Global Viral Vectors and Plasmid DNA Manufacturing Market and leading companies. You will find data, trends and predictions.
To access the data contained in this document please email contactus@visiongain.com
Buy our report today Viral Vectors & Plasmid DNA Manufacturing Market Report 2021-2031: Forecasts by Vector Types (Adenovirus, Retrovirus, Plasmid DNA, AAV, Lentivirus, Others), Application (Antisense & RNAi, Gene Therapy, Cell Therapy, Vaccinology), Disease (Oncology, Genetic Disorders, Infectious Diseases, Others), End-use (Pharma and Biopharma Companies, Research Institutes), by Region (North America, Europe, Asia Pacific, Latin America, Middle East & Africa). PLUS Analysis of Leading Viral Vectors & Plasmid DNA Manufacturing Companies AND COVID-19 Recovery Scenarios. Avoid missing out by staying informed – order our report now.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for a specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: sara.peerun@visiongain.com
1.1 Introduction to Viral Vectors and Plasmid DNA Manufacturing Market
1.2 Why You Should Read This Report
1.3 What This Report Delivers
1.4 Key Questions Answered By This Analytical Report Include:
1.5 Who is This Report For?
1.6 Methodology
1.6.1 COVID-19 Impact: Recovery Scenarios
1.6.2 Market Evaluation & Forecasting Methodology
1.7 Frequently Asked Questions (FAQs)
1.8 Associated Visiongain Reports
1.9 About Visiongain
2 Executive Summary
3 Industry Insights
3.1 Drivers
3.1.1 Robust Pipeline for Gene Therapy and Viral Vectors
3.1.2 Increasing Capacities by Manufacturers Owing to Rising Demand
3.2 Restraints
3.2.1 High Cost of Therapies
3.2.2 Bottlenecks Experienced by Manufacturers
3.3 Opportunities
3.3.1 Rise in the Development of Allogenic and Autologous Cell Therapy
3.3.2 Lucrative opportunity in manufacturing of COVID-19 vaccine
3.3.3 Increasing Applications As Preclinical Tool In Neuroscience Research
3.4 Strategies to Overcome Challenges
3.5 Porter’s Five Forces Analysis
3.5.1 Buyer Power
3.5.2 Supplier Power
3.5.3 Competitive Rivalry
3.5.4 Threat of Substitute
3.5.5 Threat of New Entrants
3.6 SWOT Analysis
3.6.1 Strengths
3.6.2 Weaknesses
3.6.3 Opportunities
3.6.4 Threats
4 Viral Vector Production Capacity Mapping Analysis
4.1 CMOs Capacity for Viral Vector Manufacturing
4.2 Cleanroom Suites Facilities: Leading Viral Vector Manufacturers
5 Viral Vector Production & Yield Analysis
5.1 Introduction
5.2 ATMP Vectors and Manufacturing Platforms
5.3 Regulatory Challenges for Viral Vectors for Cell and Gene Therapy
6 Viral Vector Manufacturing: Process Economic Considerations and Challenges
6.1 Technological Advances in Manufacturing
6.2 Stable Producer Cell Lines
6.3 Addressing the Challenges of Cytotoxicity with Stable Producer Lines
6.4 What are the Process Development Considerations for the Use of Stable Producer Lines?
6.5 How Do Stable Producer Cell Lines Create Efficiencies and Reduce Costs in the Production of CGTs?
6.5.1 Simplified Upstream Production
6.5.2 Lower Costs for Reagents and Labour
6.5.3 More Flexibility for Scale Up
6.6 When is the Time to Switch from Transient Transfection to Stable Producer Cell Lines for LVV Manufacturing?
6.7 Cost Considerations
6.8 Regulatory Expectations
7 Viral Vectors Production Process Analysis
7.1 Introduction
7.2 Upstream Unit Operations (Cell Thaw and Expansion Through Transfection)
7.3 Downstream Unit Operations (Harvest Through Purification)
7.4 Formulation/Stability and Fill/Finish
7.5 Viral Analytics
7.6 Viral Vector Production: Facilities Needs
7.7 Regulatory Standard Needs
7.8 Workforce Development and Needs
8 Viral Vectors and Plasmid DNA Manufacturing Market by Vector Types
8.1 Adenovirus Segment Market Forecast, 2021-2031
8.1.1 Introduction
8.1.2 Adenoviruses are Promising Vaccine Adjuvant
8.1.3 Recovery Scenarios (V, U, W, L)
8.2 Retrovirus Segment Market Forecast, 2021-2031
8.2.1 Introduction
8.2.2 Recovery Scenarios (V, U, W, L)
8.3 Plasmid DNA Segment Market Forecast, 2021-2031
8.3.1 Introduction
8.3.2 Recovery Scenarios (V, U, W, L)
8.4 AAV Segment Market Forecast, 2021-2031
8.4.1 Introduction
8.4.2 Recovery Scenarios (V, U, W, L)
8.5 Lentivirus Segment Market Forecast, 2021-2031
8.5.1 Introduction
8.5.2 Recovery Scenarios (V, U, W, L)
8.6 Others Segment Market Forecast, 2021-2031
8.6.1 Recovery Scenarios (V, U, W, L)
9 Viral Vectors and Plasmid DNA Manufacturing Market by Application
9.1 Antisense & RNAi Segment Market Forecast, 2021-2031
9.1.1 Introduction
9.1.2 Recovery Scenarios (V, U, W, L)
9.2 Gene Therapy Segment Market Forecast, 2021-2031
9.2.1 Introduction
9.2.2 Recovery Scenarios (V, U, W, L)
9.3 Cell Therapy Segment Market Forecast, 2021-2031
9.3.1 Introduction
9.3.2 Recovery Scenarios (V, U, W, L)
9.4 Vaccinology Segment Market Forecast, 2021-2031
9.4.1 Introduction
9.4.2 Recovery Scenarios (V, U, W, L)
10 Viral Vectors and Plasmid DNA Manufacturing Market by Disease
10.1 Oncology Segment Market Forecast, 2021-2031
10.1.1 Introduction
10.1.2 Recovery Scenarios (V, U, W, L)
10.2 Genetic Disorders Segment Market Forecast, 2021-2031
10.2.1 Introduction
10.2.2 Recovery Scenarios (V, U, W, L)
10.3 Infectious Diseases Segment Market Forecast, 2021-2031
10.3.1 Introduction
10.3.2 Recovery Scenarios (V, U, W, L)
10.4 Others Segment Market Forecast, 2021-2031
10.4.1 Recovery Scenarios (V, U, W, L)
11 Viral Vectors and Plasmid DNA Manufacturing Market by End-Use
11.1 Pharma and Biopharma Companies Segment Market Forecast, 2021-2031
11.1.1 Introduction
11.1.2 Recovery Scenarios (V, U, W, L)
11.2 Research Institutes Segment Market Forecast, 2021-2031
11.2.1 Introduction
11.2.2 Recovery Scenarios (V, U, W, L)
12 Regional and Leading National Viral Vectors and Plasmid DNA Manufacturing Market Forecasts 2021-2031
12.1 Global Viral Vectors and Plasmid DNA Manufacturing Market by Region Forecast 2021-2031
12.2 Recovery Scenarios (V, U, W, L)
13 North America Viral Vectors and Plasmid DNA Manufacturing Market
13.1 North America Viral Vectors and Plasmid DNA Manufacturing Market by Country Forecast 2021-2031
13.1.1 Recovery Scenarios (V, U, W, L)
13.2 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
13.2.1 Recovery Scenarios (V, U, W, L): North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
13.3 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
13.3.1 Recovery Scenarios (V, U, W, L): North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
13.4 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
13.4.1 Recovery Scenarios (V, U, W, L): North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
13.5 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
13.5.1 Recovery Scenarios (V, U, W, L): North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
13.6 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
13.6.1 Recovery Scenarios (V, U, W, L): U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
13.7 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
13.8 U.S. Viral Vectors and Plasmid DNA Manufacturing
13.9 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
13.10 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
13.11 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
13.12 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
13.12.1 Introduction
13.12.2 Recovery Scenarios (V, U, W, L): Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
13.13 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
13.14 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
13.15 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
13.16 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
14 Europe Viral Vectors and Plasmid DNA Manufacturing Market
14.1 Europe Viral Vectors and Plasmid DNA Manufacturing Market by Country Forecast 2021-2031
14.1.1 Recovery Scenarios (V, U, W, L)
14.2 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
14.2.1 Recovery Scenarios (V, U, W, L): Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
14.3 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
14.3.1 Recovery Scenarios (V, U, W, L): Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
14.4 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
14.4.1 Recovery Scenarios (V, U, W, L): Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
14.5 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
14.5.1 Recovery Scenarios (V, U, W, L): Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
14.6 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
14.6.1 Introduction
14.6.2 Recovery Scenarios (V, U, W, L): Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
14.7 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
14.8 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
14.9 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
14.10 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
14.11 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
14.11.1 Introduction
14.11.2 Future Capacity and Expansion in UK
14.11.3 Recovery Scenarios (V, U, W, L): UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
14.12 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
14.13 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
14.14 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
14.15 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
14.16 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
14.16.1 Recovery Scenarios (V, U, W, L): Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
14.17 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
14.18 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
14.19 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
14.20 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
15 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market
15.1 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market by Country Forecast 2021-2031
15.1.1 Recovery Scenarios (V, U, W, L)
15.2 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
15.2.1 Recovery Scenarios (V, U, W, L): Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
15.3 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
15.3.1 Recovery Scenarios (V, U, W, L): Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
15.4 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
15.4.1 Recovery Scenarios (V, U, W, L): Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
15.5 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
15.5.1 Recovery Scenarios (V, U, W, L): Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
15.6 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
15.6.1 Announcement of GenScript to Operate Plasmid Facility in China
15.6.2 Recovery Scenarios (V, U, W, L): China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
15.7 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
15.8 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
15.9 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
15.10 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
15.11 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
15.11.1 Japan is the Third Biggest Pharmaceutical Industry Globally
15.11.2 Recovery Scenarios (V, U, W, L): Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
15.12 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
15.13 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
15.14 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
15.15 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
15.16 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
15.16.1 India to Witness the Highest Growth Rate Owing to Rising Government Support Towards Biotech Sector
15.16.2 Recovery Scenarios (V, U, W, L): India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
15.17 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
15.18 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
15.19 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
15.20 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
15.21 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
15.21.1 Recovery Scenarios (V, U, W, L): Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
15.22 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
15.23 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
15.24 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
15.25 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
16 Latin America Viral Vectors and Plasmid DNA Manufacturing Market
16.1 Latin America Viral Vectors and Plasmid DNA Manufacturing Market by Country Forecast 2021-2031
16.1.1 Recovery Scenarios (V, U, W, L)
16.2 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
16.2.1 Recovery Scenarios (V, U, W, L): Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
16.3 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
16.3.1 Recovery Scenarios (V, U, W, L): Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
16.4 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
16.4.1 Recovery Scenarios (V, U, W, L): Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
16.5 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
16.5.1 Recovery Scenarios (V, U, W, L): Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
16.6 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
16.6.1 Brazil Became First Country in Latin America to Approve Commercialization of Gene Therapy Products
16.6.2 Recovery Scenarios (V, U, W, L): Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
16.7 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
16.8 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
16.9 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
16.10 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
16.11 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
16.11.1 Recovery Scenarios (V, U, W, L): Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
16.12 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
16.13 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
16.14 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
16.15 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
17 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market
17.1 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market by Country Forecast 2021-2031
17.1.1 Recovery Scenarios (V, U, W, L)
17.2 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
17.2.1 Recovery Scenarios (V, U, W, L): Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
17.3 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
17.3.1 Recovery Scenarios (V, U, W, L): Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
17.4 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
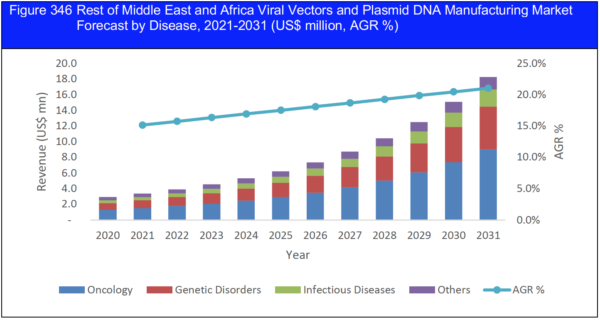
17.4.1 Recovery Scenarios (V, U, W, L): Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
17.5 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
17.5.1 Recovery Scenarios (V, U, W, L): Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
17.6 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
17.6.1 South African Government Focussing to Strengthen Biotech Industry
17.6.2 Recovery Scenarios (V, U, W, L): South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
17.7 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
17.8 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
17.9 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
17.10 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
17.11 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
17.11.1 Recovery Scenarios (V, U, W, L): Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031
17.12 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031
17.13 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031
17.14 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031
17.15 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031
18 Company Profiles
18.1 Merck KGaA
18.1.1 Company Snapshot, 2020
18.1.2 Company Overview
18.1.3 Financial Analysis, 2015-2019
18.1.4 Product Benchmarking
18.1.5 Recent Developments
18.2 Lonza
18.2.1 Company Snapshot, 2020
18.2.2 Company Overview
18.2.3 Financial Analysis, 2015-2019
18.2.4 Product Benchmarking
18.2.5 Recent Developments
18.3 FUJIFILM Diosynth Biotechnologies U.S.A., Inc
18.3.1 Company Snapshot, 2020
18.3.2 Company Overview
18.3.3 Financial Analysis, 2015-2019
18.3.4 Product Benchmarking
18.3.5 Recent Developments
18.4 Cobra Biologics Ltd.
18.4.1 Company Snapshot, 2020
18.4.2 Company Overview
18.4.3 Product Benchmarking
18.4.4 Recent Developments
18.5 Brammer Bio
18.5.1 Company Snapshot, 2020
18.5.2 Company Overview
18.5.3 Product Benchmarking
18.5.4 Recent Developments
18.6 Waisman Biomanufacturing
18.6.1 Company Snapshot, 2020
18.6.2 Company Overview
18.6.3 Product Benchmarking
18.7 Genezen
18.7.1 Company Snapshot, 2020
18.7.2 Company Overview
18.7.3 Product Benchmarking
18.7.4 Recent Developments
18.8 YPOSKESI
18.8.1 Company Snapshot, 2020
18.8.2 Company Overview
18.8.3 Product Benchmarking
18.8.4 Recent Developments
18.9 Advanced BioScience Laboratories, Inc. (ABL, Inc.)
18.9.1 Company Snapshot, 2020
18.9.2 Company Overview
18.9.3 Product Benchmarking
18.9.4 Recent Developments
18.10 Novasep Holding S.A.S
18.10.1 Company Snapshot, 2020
18.10.2 Company Overview
18.10.3 Product Benchmarking
18.10.4 Recent Developments
18.11 ATVIO Biotech Ltd
18.11.1 Company Snapshot, 2020
18.11.2 Company Overview
18.11.3 Product Benchmarking
18.11.4 Recent Developments
18.12 Vigene Biosciences, Inc
18.12.1 Company Snapshot, 2020
18.12.2 Company Overview
18.12.3 Product Benchmarking
18.12.4 Recent Developments
18.13 Cytiva
18.13.1 Company Snapshot, 2020
18.13.2 Company Overview
18.13.3 Financial Analysis, 2015-2019
18.13.4 Recent Developments
18.14 CEVEC Pharmaceuticals GmbH
18.14.1 Company Snapshot, 2020
18.14.2 Company Overview
18.14.3 Product Benchmarking
18.14.4 Recent Developments
18.15 Batavia Biosciences B.V
18.15.1 Company Snapshot, 2020
18.15.2 Company Overview
18.15.3 Product Benchmarking
18.15.4 Recent Developments
18.16 Biovian Oy
18.16.1 Company Snapshot, 2020
18.16.2 Company Overview
18.16.3 Product Benchmarking
18.17 Wuxi AppTec Co., Ltd.
18.17.1 Company Snapshot, 2020
18.17.2 Company Overview
18.17.3 Product Benchmarking
18.17.4 Recent Developments
18.18 VGXI, Inc.
18.18.1 Company Snapshot, 2020
18.18.2 Company Overview
18.18.3 Product Benchmarking
18.19 Paragon Bioservices, Inc.
18.19.1 Company Snapshot, 2020
18.19.2 Company Overview
18.19.3 Product Benchmarking
18.19.4 Recent Developments
18.20 Miltenyi Biotec GmbH
18.20.1 Company Snapshot, 2020
18.20.2 Company Overview
18.21 SIRION Biotech GmbH
18.21.1 Company Snapshot, 2020
18.21.2 Company Overview
18.21.3 Product Benchmarking
18.22 Virovek Incorporation
18.22.1 Company Snapshot, 2020
18.22.2 Company Overview
18.22.3 Product Benchmarking
18.23 BioNTech IMFS GmbH
18.23.1 Company Snapshot, 2020
18.23.2 Company Overview
18.23.3 Product Benchmarking
18.24 VIVEbiotech S.L.
18.24.1 Company Snapshot, 2020
18.24.2 Company Overview
18.24.3 Product Benchmarking
18.24.4 Recent Developments
18.25 Creative Biogene
18.25.1 Company Snapshot, 2020
18.25.2 Company Overview
18.25.3 Product Benchmarking
18.25.4 Recent Developments
18.26 Vibalogics GmbH
18.26.1 Company Snapshot, 2020
18.26.2 Company Overview
18.26.3 Product Benchmarking
18.26.4 Recent Developments
18.27 Cell and Gene Therapy Catapult
18.27.1 Company Snapshot, 2020
18.27.2 Company Overview
18.28 BlueBird Bio
18.28.1 Company Snapshot, 2020
18.28.2 Company Overview
18.29 Addgene, Inc.
18.29.1 Company Snapshot, 2020
18.29.2 Company Overview
18.29.3 Product Benchmarking
18.29.4 Recent Developments
18.30 Aldevron, L.L.C.
18.30.1 Company Snapshot, 2020
18.30.2 Company Overview
18.30.3 Product Benchmarking
18.31 Audentes Therapeutics
18.31.1 Company Snapshot, 2020
18.31.2 Company Overview
18.31.3 Recent Developments
18.32 BioMarin Pharmaceutical
18.32.1 Company Snapshot, 2020
18.32.2 Company Overview
18.32.3 Financial Analysis, 2015-2019
18.32.4 Recent Developments
19 Conclusion
19.1 Concluding Remarks
19.2 Choosing a Cell Culture Technology
19.3 Increased Urgency In Addressing Manufacturing Challenges
List of Tables
Table 1 Global Viral Vectors and Plasmid DNA Manufacturing Market Snapshot, 2020 & 2031 (US$ million, CAGR %)
Table 2 Advantages and Disadvantages of Adeno-Associated Virus Vectors
Table 3 Key Factors Affecting the Bargaining Power of Buyer
Table 4 Key Factors Affecting the Bargaining Power of Suppliers
Table 5 Key Factors Affecting the Industry Rivalry
Table 6 Key Factors Affecting the Threat of Substitutes
Table 7 Key Factors Affecting the Threat of New Entrants
Table 8 CMOs Capacity for Viral Vector Manufacturing
Table 9 Cleanroom Suites Facilities
Table 1 Global Viral Vectors and Plasmid DNA Manufacturing Market for Adenovirus Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 2 Global Viral Vectors and Plasmid DNA Manufacturing Market for Adenovirus Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 3 Global Viral Vectors and Plasmid DNA Manufacturing Market for Adenovirus Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 4 Global Viral Vectors and Plasmid DNA Manufacturing Market for Adenovirus Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 5 Global Viral Vectors and Plasmid DNA Manufacturing Market for Adenovirus Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 6 Global Viral Vectors and Plasmid DNA Manufacturing Market for Retrovirus Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 7 Global Viral Vectors and Plasmid DNA Manufacturing Market for Retrovirus Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 8 Global Viral Vectors and Plasmid DNA Manufacturing Market for Retrovirus Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 9 Global Viral Vectors and Plasmid DNA Manufacturing Market for Retrovirus Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 10 Global Viral Vectors and Plasmid DNA Manufacturing Market for Retrovirus Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 11 Global Viral Vectors and Plasmid DNA Manufacturing Market for Plasmid DNA Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 12 Global Viral Vectors and Plasmid DNA Manufacturing Market for Plasmid DNA Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 13 Global Viral Vectors and Plasmid DNA Manufacturing Market for Plasmid DNA Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 14 Global Viral Vectors and Plasmid DNA Manufacturing Market for Plasmid DNA Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 15 Global Viral Vectors and Plasmid DNA Manufacturing Market for Plasmid DNA Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 16 Global Viral Vectors and Plasmid DNA Manufacturing Market for AAV Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 17 Global Viral Vectors and Plasmid DNA Manufacturing Market for AAV Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 18 Global Viral Vectors and Plasmid DNA Manufacturing Market for AAV Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 19 Global Viral Vectors and Plasmid DNA Manufacturing Market for AAV Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 20 Global Viral Vectors and Plasmid DNA Manufacturing Market for AAV Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 21 Global Viral Vectors and Plasmid DNA Manufacturing Market for Lentivirus Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 22 Global Viral Vectors and Plasmid DNA Manufacturing Market for Lentivirus Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 23 Global Viral Vectors and Plasmid DNA Manufacturing Market for Lentivirus Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 24 Global Viral Vectors and Plasmid DNA Manufacturing Market for Lentivirus Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 25 Global Viral Vectors and Plasmid DNA Manufacturing Market for Lentivirus Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 26 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 27 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 28 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 29 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 30 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 31 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 32 Global Viral Vectors and Plasmid DNA Manufacturing Market for Antisense & RNAi Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 33 Global Viral Vectors and Plasmid DNA Manufacturing Market for Antisense & RNAi Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 34 Global Viral Vectors and Plasmid DNA Manufacturing Market for Antisense & RNAi Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 35 Global Viral Vectors and Plasmid DNA Manufacturing Market for Antisense & RNAi Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 36 Global Viral Vectors and Plasmid DNA Manufacturing Market for Antisense & RNAi Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 37 The current industry-wide gene therapy pipeline can be organized by stage and therapeutic area
Table 38 Global Viral Vectors and Plasmid DNA Manufacturing Market for Gene Therapy Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 39 Global Viral Vectors and Plasmid DNA Manufacturing Market for Gene Therapy Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 40 Global Viral Vectors and Plasmid DNA Manufacturing Market for Gene Therapy Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 41 Global Viral Vectors and Plasmid DNA Manufacturing Market for Gene Therapy Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 42 Global Viral Vectors and Plasmid DNA Manufacturing Market for Gene Therapy Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 43 List of Cell Therapy Companies Worldwide by Location and Type
Table 44 Global Viral Vectors and Plasmid DNA Manufacturing Market for Cell Therapy Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 45 Global Viral Vectors and Plasmid DNA Manufacturing Market for Cell Therapy Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 46 Global Viral Vectors and Plasmid DNA Manufacturing Market for Cell Therapy Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 47 Global Viral Vectors and Plasmid DNA Manufacturing Market for Cell Therapy Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 48 Global Viral Vectors and Plasmid DNA Manufacturing Market for Cell Therapy Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 49 Global Viral Vectors and Plasmid DNA Manufacturing Market for Vaccinology Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 50 Global Viral Vectors and Plasmid DNA Manufacturing Market for Vaccinology Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 51 Global Viral Vectors and Plasmid DNA Manufacturing Market for Vaccinology Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 52 Global Viral Vectors and Plasmid DNA Manufacturing Market for Vaccinology Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 53 Global Viral Vectors and Plasmid DNA Manufacturing Market for Vaccinology Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 54 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 55 Global Viral Vectors and Plasmid DNA Manufacturing Market for Oncology Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 56 Global Viral Vectors and Plasmid DNA Manufacturing Market for Oncology Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 57 Global Viral Vectors and Plasmid DNA Manufacturing Market for Oncology Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 58 Global Viral Vectors and Plasmid DNA Manufacturing Market for Oncology Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 59 Global Viral Vectors and Plasmid DNA Manufacturing Market for Oncology Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 60 Global Viral Vectors and Plasmid DNA Manufacturing Market for Genetic Disorders Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 61 Global Viral Vectors and Plasmid DNA Manufacturing Market for Genetic Disorders Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 62 Global Viral Vectors and Plasmid DNA Manufacturing Market for Genetic Disorders Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 63 Global Viral Vectors and Plasmid DNA Manufacturing Market for Genetic Disorders Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 64 Global Viral Vectors and Plasmid DNA Manufacturing Market for Genetic Disorders Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 65 Global Viral Vectors and Plasmid DNA Manufacturing Market for Infectious Diseases Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 66 Global Viral Vectors and Plasmid DNA Manufacturing Market for Infectious Diseases Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 67 Global Viral Vectors and Plasmid DNA Manufacturing Market for Infectious Diseases Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 68 Global Viral Vectors and Plasmid DNA Manufacturing Market for Infectious Diseases Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 69 Global Viral Vectors and Plasmid DNA Manufacturing Market for Infectious Diseases Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 70 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 71 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 72 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 73 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 74 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 75 Global Viral Vectors and Plasmid DNA Manufacturing Market for Pharma & Biopharma Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 76 Global Viral Vectors and Plasmid DNA Manufacturing Market for Pharma & Biopharma Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 77 Global Viral Vectors and Plasmid DNA Manufacturing Market for Pharma & Biopharma Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 78 Global Viral Vectors and Plasmid DNA Manufacturing Market for Pharma & Biopharma Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 79 Global Viral Vectors and Plasmid DNA Manufacturing Market for Pharma & Biopharma Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 80 Global Viral Vectors and Plasmid DNA Manufacturing Market for Research Institutes Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 81 Global Viral Vectors and Plasmid DNA Manufacturing Market for Research Institutes Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 82 Global Viral Vectors and Plasmid DNA Manufacturing Market for Research Institutes Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 83 Global Viral Vectors and Plasmid DNA Manufacturing Market for Research Institutes Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 84 Global Viral Vectors and Plasmid DNA Manufacturing Market for Research Institutes Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 85 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 86 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 87 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 88 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 89 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 90 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 91 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 92 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 93 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 94 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 95 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 96 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 97 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 98 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 99 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 100 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 101 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 102 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 103 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 104 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 105 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 106 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 107 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 108 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 109 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 110 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 111 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 112 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 113 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 114 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 115 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 116 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 117 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 118 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 119 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 120 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 121 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 122 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 123 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 124 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 125 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 126 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 127 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 128 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 129 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 130 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 131 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 132 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 133 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 134 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 135 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 136 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 137 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 138 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 139 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 140 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 141 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 142 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 143 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 144 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 145 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 146 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 147 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 148 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 149 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 150 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 151 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 152 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 153 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 154 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 155 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 156 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 157 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 158 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 159 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 160 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 161 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 162 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 163 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 164 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 165 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 166 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 167 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 168 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 169 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 170 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 171 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 172 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 173 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 174 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 175 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 176 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 177 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 178 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 179 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 180 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 181 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 182 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 183 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 184 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 185 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 186 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 187 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 188 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 189 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 190 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 191 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 192 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 193 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 194 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 195 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 196 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 197 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 198 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 199 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 200 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 201 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 202 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 203 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 204 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 205 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 206 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 207 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 208 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 209 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 210 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 211 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 212 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 213 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 214 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 215 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 216 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 217 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 218 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 219 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 220 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 221 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 222 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 223 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 224 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 225 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 226 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 227 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 228 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 229 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 230 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 231 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 232 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 233 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 234 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 235 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 236 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 237 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 238 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 239 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 240 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 241 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 242 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 243 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 244 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 245 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 246 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 247 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 248 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 249 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 250 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 251 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 252 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 253 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 254 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 255 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 256 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 257 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 258 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 259 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 260 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 261 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 262 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 263 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 264 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 265 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 266 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 267 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 268 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 269 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 270 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 271 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 272 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 273 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 274 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 275 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 276 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 277 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 278 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 279 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 280 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 281 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 282 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 283 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 284 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 285 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 286 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 287 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 288 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 289 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 290 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 291 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 292 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 293 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 294 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 295 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 296 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 297 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 298 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 299 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 300 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 301 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 302 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 303 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 304 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 305 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 306 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 307 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 308 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 309 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 310 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 311 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 312 MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 313 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 314 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 315 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 316 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 317 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 318 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 319 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 320 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 321 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 322 Rest of MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ Mn, AGR%, CAGR%)
Table 323 Rest of MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "V" Shaped Recovery
Table 324 Rest of MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "U" Shaped Recovery
Table 325 Rest of MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "W" Shaped Recovery
Table 326 Rest of MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ mn, AGR%, CAGR%): "L" Shaped Recovery
Table 327 Rest of MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Type, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 328 Rest of MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 329 Rest of MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 330 Rest of MEA Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ mn, AGR%, CAGR%)
Table 331 Merck KGaA: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 332 Merck KGaA: Product Portfolio
Table 333 Merck KGaA: Recent Developments
Table 334 Lonza: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 335 Lonza: Product Portfolio
Table 336 Lonza: Recent Developments
Table 337 FUJIFILM Diosynth Biotechnologies U.S.A., Inc: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 338 FUJIFILM Diosynth Biotechnologies U.S.A., Inc: Product Portfolio
Table 339 FUJIFILM Diosynth Biotechnologies U.S.A., Inc: Recent Developments
Table 340 Cobra Biologics Ltd.: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 341 Cobra Biologics Ltd.: Product Portfolio
Table 342 Cobra Biologics Ltd.: Recent Developments
Table 343 Brammer Bio: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 344 Brammer Bio: Product Portfolio
Table 345 Brammer Bio: Recent Developments
Table 346 Waisman Biomanufacturing: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 347 Waisman Biomanufacturing: Product Portfolio
Table 348 Genezen: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 349 Genezen: Product Portfolio
Table 350 Genezen: Recent Developments
Table 351 YPOSKESI: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 352 YPOSKESI: Product Portfolio
Table 353 YPOSKESI: Recent Developments
Table 354 Advanced BioScience Laboratories, Inc. (ABL, Inc.): Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 355 Advanced BioScience Laboratories, Inc. (ABL, Inc.): Product Portfolio
Table 356 Advanced BioScience Laboratories, Inc. (ABL, Inc.): Recent Developments
Table 357 Novasep Holding S.A.S: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 358 Novasep Holding S.A.S: Product Portfolio
Table 359 Novasep Holding S.A.S: Recent Developments
Table 360 ATVIO Biotech Ltd: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 361 ATVIO Biotech Ltd: Product Portfolio
Table 362 ATVIO Biotech Ltd: Recent Developments
Table 363 Vigene Biosciences, Inc: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 364 Vigene Biosciences, Inc: Product Portfolio
Table 365 Vigene Biosciences, Inc: Recent Developments
Table 366 Cytiva: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 367 Cytiva: Recent Developments
Table 368 CEVEC Pharmaceuticals GmbH: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 369 CEVEC Pharmaceuticals GmbH: Product Portfolio
Table 370 CEVEC Pharmaceuticals GmbH: Recent Developments
Table 371 Batavia Biosciences B.V: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 372 Batavia Biosciences B.V: Product Portfolio
Table 373 Batavia Biosciences B.V: Recent Developments
Table 374 Biovian Oy: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 375 Biovian Oy: Product Portfolio
Table 376 Wuxi AppTec Co., Ltd.: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 377 Wuxi AppTec Co., Ltd.: Product Portfolio
Table 378 Wuxi AppTec Co., Ltd.: Recent Developments
Table 379 VGXI, Inc.: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 380 VGXI, Inc.: Product Portfolio
Table 381 Paragon Bioservices, Inc.: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 382 Paragon Bioservices, Inc.: Product Portfolio
Table 383 Paragon Bioservices, Inc.: Recent Developments
Table 384 Miltenyi Biotec GmbH: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 385 SIRION Biotech GmbH: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 386 SIRION Biotech GmbH: Product Portfolio
Table 387 Virovek Incorporation: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 388 Virovek Incorporation: Product Portfolio
Table 389 BioNTech IMFS GmbH: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 390 BioNTech IMFS GmbH: Product Portfolio
Table 391 VIVEbiotech S.L.: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 392 VIVEbiotech S.L.: Product Portfolio
Table 393 VIVEbiotech S.L.: Recent Developments
Table 394 Creative Biogene: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 395 Creative Biogene: Product Portfolio
Table 396 Creative Biogene: Recent Developments
Table 397 Vibalogics GmbH: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 398 Vibalogics GmbH: Product Portfolio
Table 399 Vibalogics GmbH: Recent Developments
Table 400 Cell and Gene Therapy Catapult: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 401 BlueBird Bio: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 402 Addgene, Inc.: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 403 Addgene, Inc.: Product Portfolio
Table 404 Addgene, Inc.: Recent Developments
Table 405 Aldevron, L.L.C.: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 406 Aldevron, L.L.C.: Product Portfolio
Table 407 Audentes Therapeutics: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 408 Audentes Therapeutics: Recent Developments
Table 409 BioMarin Pharmaceutical: Key Details, 2020 (CEO, HQ, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 410 BioMarin Pharmaceutical: Recent Developments
List of Figures
Figure 1 Global Viral Vectors and Plasmid DNA Manufacturing Market: Market Trends
Figure 2 Cell and gene therapy modalities
Figure 3 COVID-19 vaccine pipeline by type
Figure 4 Porter’s Analysis
Figure 5 Upstream Analysis
Figure 6 Downstream Analysis
Figure 7 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 8 Global Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Vector Types, 2021, 2026, 2031 (%)
Figure 9 Global Viral Vectors and Plasmid DNA Manufacturing Market for Adenovirus Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 10 Global Viral Vectors and Plasmid DNA Manufacturing Market for Adenovirus Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 11 Global Viral Vectors and Plasmid DNA Manufacturing Market for Adenovirus Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 12 Global Viral Vectors and Plasmid DNA Manufacturing Market for Adenovirus Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 13 Global Viral Vectors and Plasmid DNA Manufacturing Market for Adenovirus Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 14 Global Viral Vectors and Plasmid DNA Manufacturing Market for Retrovirus Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 15 Global Viral Vectors and Plasmid DNA Manufacturing Market for Retrovirus Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 16 Global Viral Vectors and Plasmid DNA Manufacturing Market for Retrovirus Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 17 Global Viral Vectors and Plasmid DNA Manufacturing Market for Retrovirus Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 18 Global Viral Vectors and Plasmid DNA Manufacturing Market for Retrovirus Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 19 Global Viral Vectors and Plasmid DNA Manufacturing Market for Plasmid DNA Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 20 Global Viral Vectors and Plasmid DNA Manufacturing Market for Plasmid DNA Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 21 Global Viral Vectors and Plasmid DNA Manufacturing Market for Plasmid DNA Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 22 Global Viral Vectors and Plasmid DNA Manufacturing Market for Plasmid DNA Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 23 Global Viral Vectors and Plasmid DNA Manufacturing Market for Plasmid DNA Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 24 Global Viral Vectors and Plasmid DNA Manufacturing Market for AAV Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 25 Global Viral Vectors and Plasmid DNA Manufacturing Market for AAV Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 26 Global Viral Vectors and Plasmid DNA Manufacturing Market for AAV Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 27 Global Viral Vectors and Plasmid DNA Manufacturing Market for AAV Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 28 Global Viral Vectors and Plasmid DNA Manufacturing Market for AAV Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 29 Global Viral Vectors and Plasmid DNA Manufacturing Market for Lentivirus Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 30 Global Viral Vectors and Plasmid DNA Manufacturing Market for Lentivirus Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 31 Global Viral Vectors and Plasmid DNA Manufacturing Market for Lentivirus Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 32 Global Viral Vectors and Plasmid DNA Manufacturing Market for Lentivirus Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 33 Global Viral Vectors and Plasmid DNA Manufacturing Market for Lentivirus Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 34 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 35 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 36 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 37 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 38 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 39 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 40 Global Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Vector Types, 2021, 2026, 2031 (%)
Figure 41 Global Viral Vectors and Plasmid DNA Manufacturing Market for Antisense & RNAi Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 42 Global Viral Vectors and Plasmid DNA Manufacturing Market for Antisense & RNAi Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 43 Global Viral Vectors and Plasmid DNA Manufacturing Market for Antisense & RNAi Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 44 Global Viral Vectors and Plasmid DNA Manufacturing Market for Antisense & RNAi Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 45 Global Viral Vectors and Plasmid DNA Manufacturing Market for Antisense & RNAi Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 46 Global Viral Vectors and Plasmid DNA Manufacturing Market for Gene Therapy Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 47 Global Viral Vectors and Plasmid DNA Manufacturing Market for Gene Therapy Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 48 Global Viral Vectors and Plasmid DNA Manufacturing Market for Gene Therapy Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 49 Global Viral Vectors and Plasmid DNA Manufacturing Market for Gene Therapy Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 50 Global Viral Vectors and Plasmid DNA Manufacturing Market for Gene Therapy Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 51 Global Viral Vectors and Plasmid DNA Manufacturing Market for Cell Therapy Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 52 Global Viral Vectors and Plasmid DNA Manufacturing Market for Cell Therapy Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 53 Global Viral Vectors and Plasmid DNA Manufacturing Market for Cell Therapy Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 54 Global Viral Vectors and Plasmid DNA Manufacturing Market for Cell Therapy Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 55 Global Viral Vectors and Plasmid DNA Manufacturing Market for Cell Therapy Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 56 Global Viral Vectors and Plasmid DNA Manufacturing Market for Vaccinology Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 57 Global Viral Vectors and Plasmid DNA Manufacturing Market for Vaccinology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 58 Global Viral Vectors and Plasmid DNA Manufacturing Market for Vaccinology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 59 Global Viral Vectors and Plasmid DNA Manufacturing Market for Vaccinology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 60 Global Viral Vectors and Plasmid DNA Manufacturing Market for Vaccinology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 61 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 62 Global Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Disease, 2021, 2026, 2031 (%)
Figure 63 Global Viral Vectors and Plasmid DNA Manufacturing Market for Oncology Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 64 Global Viral Vectors and Plasmid DNA Manufacturing Market for Oncology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 65 Global Viral Vectors and Plasmid DNA Manufacturing Market for Oncology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 66 Global Viral Vectors and Plasmid DNA Manufacturing Market for Oncology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 67 Global Viral Vectors and Plasmid DNA Manufacturing Market for Oncology Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 68 Global Viral Vectors and Plasmid DNA Manufacturing Market for Genetic Disorders Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 69 Global Viral Vectors and Plasmid DNA Manufacturing Market for Genetic Disorders Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 70 Global Viral Vectors and Plasmid DNA Manufacturing Market for Genetic Disorders Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 71 Global Viral Vectors and Plasmid DNA Manufacturing Market for Genetic Disorders Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 72 Global Viral Vectors and Plasmid DNA Manufacturing Market for Genetic Disorders Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 73 Global Viral Vectors and Plasmid DNA Manufacturing Market for Infectious Diseases Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 74 Global Viral Vectors and Plasmid DNA Manufacturing Market for Infectious Diseases Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 75 Global Viral Vectors and Plasmid DNA Manufacturing Market for Infectious Diseases Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 76 Global Viral Vectors and Plasmid DNA Manufacturing Market for Infectious Diseases Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 77 Global Viral Vectors and Plasmid DNA Manufacturing Market for Infectious Diseases Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 78 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 79 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 80 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 81 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 82 Global Viral Vectors and Plasmid DNA Manufacturing Market for Others Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 83 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 84 Global Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by End-Use, 2021, 2026, 2031 (%)
Figure 85 Global Viral Vectors and Plasmid DNA Manufacturing Market for Pharma and Biopharma Companies Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 86 Global Viral Vectors and Plasmid DNA Manufacturing Market for Pharma and Biopharma Companies Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 87 Global Viral Vectors and Plasmid DNA Manufacturing Market for Pharma and Biopharma Companies Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 88 Global Viral Vectors and Plasmid DNA Manufacturing Market for Pharma and Biopharma Companies Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 89 Global Viral Vectors and Plasmid DNA Manufacturing Market for Pharma and Biopharma Companies Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 90 Global Viral Vectors and Plasmid DNA Manufacturing Market for Research Institutes Segment: Revenue Forecast 2021-2031 (US$ million, AGR%)
Figure 91 Global Viral Vectors and Plasmid DNA Manufacturing Market for Research Institutes Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 92 Global Viral Vectors and Plasmid DNA Manufacturing Market for Research Institutes Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 93 Global Viral Vectors and Plasmid DNA Manufacturing Market for Research Institutes Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 94 Global Viral Vectors and Plasmid DNA Manufacturing Market for Research Institutes Segment, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 95 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Region 2021-2031 (US$ million)
Figure 96 Global Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Region 2021, 2026, 2031 (%)
Figure 97 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 98 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 99 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 100 Global Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 101 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country 2021-2031 (US$ million)
Figure 102 North America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Country 2021, 2026, 2031 (%)
Figure 103 North America Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 104 North America Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 105 North America Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 106 North America Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 107 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 108 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 109 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 110 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 111 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 112 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 113 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 114 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 115 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 116 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 117 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 118 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 119 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 120 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 121 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 122 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 123 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 124 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 125 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 126 North America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 127 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 128 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 129 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 130 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 131 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 132 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 133 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 134 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 135 U.S. Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 136 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 137 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 138 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 139 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 140 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 141 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 142 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 143 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 144 Canada Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 145 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country 2021-2031 (US$ million)
Figure 146 Europe Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Country 2021, 2026, 2031 (%)
Figure 147 Europe Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 148 Europe Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 149 Europe Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 150 Europe Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 151 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 152 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 153 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 154 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 155 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 156 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 157 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 158 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 159 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 160 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 161 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 162 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 163 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 164 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 165 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 166 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 167 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 168 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 169 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 170 Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 171 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 172 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 173 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 174 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 175 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 176 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 177 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 178 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 179 Germany Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 180 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 181 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 182 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 183 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 184 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 185 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 186 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 187 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 188 UK Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 189 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 190 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 191 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 192 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 193 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 194 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 195 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 196 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 197 Rest of Europe Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 198 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country 2021-2031 (US$ million)
Figure 199 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Country 2021, 2026, 2031 (%)
Figure 200 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 201 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 202 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 203 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 204 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 205 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 206 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 207 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 208 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 209 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 210 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 211 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 212 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 213 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 214 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 215 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 216 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 217 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 218 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 219 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 220 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 221 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 222 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 223 Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 224 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 225 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 226 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 227 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 228 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 229 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 230 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 231 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 232 China Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 233 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 234 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 235 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 236 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 237 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 238 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 239 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 240 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 241 Japan Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 242 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 243 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 244 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 245 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 246 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 247 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 248 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 249 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 250 India Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 251 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 252 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 253 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 254 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 255 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 256 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 257 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 258 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 259 Rest of Asia Pacific Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 260 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country 2021-2031 (US$ million)
Figure 261 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Country 2021, 2026, 2031 (%)
Figure 262 Latin America Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 263 Latin America Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 264 Latin America Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 265 Latin America Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 266 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 267 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 268 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 269 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 270 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 271 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 272 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 273 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 274 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 275 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 276 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 277 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 278 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 279 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 280 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 281 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 282 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 283 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 284 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 285 Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 286 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 287 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 288 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 289 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 290 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 291 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 292 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 293 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 294 Brazil Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 295 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 296 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 297 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 298 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 299 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 300 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 301 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 302 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 303 Rest of Latin America Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 304 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Country 2021-2031 (US$ million)
Figure 305 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Share Forecast by Country 2021, 2026, 2031 (%)
Figure 306 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 307 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 308 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 309 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market by Country, Revenue Forecast 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 310 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 311 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 312 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 313 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 314 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 315 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 316 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 317 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 318 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 319 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 320 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 321 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 322 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 323 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 324 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 325 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 326 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 327 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 328 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 329 Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 330 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 331 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 332 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 333 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 334 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 335 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 336 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 337 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 338 South Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 339 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR %)
Figure 340 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “V” Shaped Recovery
Figure 341 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “U” Shaped Recovery
Figure 342 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “W” Shaped Recovery
Figure 343 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast, 2021-2031 (US$ million, AGR%): “L” Shaped Recovery
Figure 344 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Vector Types, 2021-2031 (US$ million, AGR %)
Figure 345 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Application, 2021-2031 (US$ million, AGR %)
Figure 346 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by Disease, 2021-2031 (US$ million, AGR %)
Figure 347 Rest of Middle East and Africa Viral Vectors and Plasmid DNA Manufacturing Market Forecast by End-Use, 2021-2031 (US$ million, AGR %)
Figure 348 Merck KGaA.: Net Revenue, 2015-2019 (US$ Mn, AGR%)
Figure 349 Lonza.: Net Revenue, 2015-2019 (US$ Mn, AGR%)
Figure 350 FUJIFILM Diosynth Biotechnologies U.S.A., Inc.: Net Revenue, 2015-2019 (US$ Mn, AGR%)
Figure 351 BioMarin Pharmaceutical.: Net Revenue, 2015-2019 (US$ Mn, AGR%)
Addgene, Inc.
Advanced BioScience Laboratories, Inc. (ABL, Inc.)
Aldevron, L.L.C.
ATVIO Biotech Ltd
Audentes Therapeutics
Batavia Biosciences B.V
BioMarin Pharmaceutical
BioNTech IMFS GmbH
Biovian Oy
BlueBird Bio
Brammer Bio
Cell and Gene Therapy Catapult
CEVEC Pharmaceuticals GmbH
Cobra Biologics Ltd.
Creative Biogene
Cytiva
FUJIFILM Diosynth Biotechnologies U.S.A., Inc.
Genezen
Lonza
Merck KGaA
Miltenyi Biotec GmbH
Novasep Holding S.A.S
Paragon Bioservices, Inc.
SIRION Biotech GmbH
VGXI, Inc.
Vibalogics GmbH
Vigene Biosciences, Inc.
Virovek Incorporation
VIVEbiotech S.L.
Waisman Biomanufacturing
Wuxi AppTec Co., Ltd.
YPOSKESI
List of Other Companies
Abeona Therapeutics
Acucela
Adaptimmune Therapeutics
Autolus
AveXis
BioCancell
Biomay
Biomiga
BioReliance
Biotec Services International
Celladon
Cellectis
Cellular Biomedicine Group
Delphi Genetics
Department of Neuroscience, University of Minnesota
Desktop Genetics
DNAtrix
Elixirgen Scientific
Epeius Biotechnologies
EUFETS
Eurofins Genomics
European Society of Gene and Cell Therapy
GEG Tech
Genable Technologies
Immune Design
Immune Technology
ImmunoGenes
Immunomic Therapeutics
Inbiomed
Indiana University Vector Production Facility
VIVEbiotech
Voyager Therapeutics
Waisman Biomanufacturing
Xpress Biologics
List of Organizations Mentioned in the Report
c-LEcta GmbH
Gene Therapy Net
Informa UK Limited
IntechOpen
JOURNAL OF GENERAL VIROLOGY
Massachusetts Medical Society
National Center for Biotechnology Information
Pan European Networks Ltd
Pharma Manufacturing
Pharmaceutical-Networking.com
The Alliance for Regenerative Medicine
The Royal Society
Transgene
University of Rochester
x Labiotech UG
Download sample pages
Complete the form below to download your free sample pages for Viral Vectors & Plasmid DNA Manufacturing Market Report 2021-2031
Related reports
-

Track and Trace Solutions Market Report 2021-2031
The key factors driving the growth of this market include the severe regulations & principles for the implementation of serialization,...
Full DetailsPublished: 01 January 1970 -

Rare Diseases Market Report 2021-2031
Increasing cases of rare genetic diseases coupled with rising number of US FDA approvals for treatment of rare diseases is...Full DetailsPublished: 01 March 2021 -

ePharmacy Market Report 2021-2031
The growth in online shopping has been driven by a rapid rise in the number of smartphones, broadband connections and...Full DetailsPublished: 19 April 2021 -

Global Bioprocess Optimisation & Digital Biomanufacturing Market Forecast to 2029
The global market for bioprocess optimisation and digital biomanufacturing was valued to be $29.7bn in 2019 and is valued to...
Full DetailsPublished: 28 February 2020 -

Drugs of Abuse Testing Market Report 2021-2031
Drugs Abuse has risen very significantly in few last year as compared to previous once and expected to continuously rise...Full DetailsPublished: 01 January 1970 -

Virus Testing Diagnostic Kits Market Report 2021-2031
With the growing burden of rare diseases around the world, there has been a rise in the requirement for proper...Full DetailsPublished: 25 November 2020 -

Gene Therapy R&D Market Report 2021-2031
Our new study lets you assess forecasted sales at overall world market and regional level. See financial results, trends, opportunities,...Full DetailsPublished: 11 June 2021 -

Plasma Therapy Market Report 2021-2031
The market is forecasted for the next ten years based on market growth within each segment of the blood plasma...Full DetailsPublished: 16 November 2020 -

Next-Generation Pharmaceutical Temperature Management Solutions Market Report 2020-2030
Maintaining the quality and safety of the product in the supply chain has always been a high priority for pharmaceutical...Full DetailsPublished: 25 September 2020 -

Global Clinical Trial Management System Market Forecast 2020-2030
The global market for clinical trial management systems was valued to be $1,152m in 2019 and is valued to reach...
Full DetailsPublished: 17 March 2020
Download sample pages
Complete the form below to download your free sample pages for Viral Vectors & Plasmid DNA Manufacturing Market Report 2021-2031
Do you have any custom requirements we can help you with?
Any specific country, geo region, market segment or specific company information?
Email us today, we can discuss your needs and see how we can help: jamie.roberts@visiongain.com
Would you like a free report overview of the report of your choice?
If so, please drop an email to Jamie Roberts stating your chosen report title to jamie.roberts@visiongain.com
Visiongain’s reports are based on comprehensive primary and secondary research. Those studies provide global market forecasts (sales by drug and class, with sub-markets and leading nations covered) and analyses of market drivers and restraints (including SWOT analysis) and current pipeline developments. To find out more about our reports methodology, please email jamie.roberts@visiongain.com
“Thank you for this Gene Therapy R&D Market report and for how easy the process was. Your colleague was very helpful and the report is just right for my purpose. This is the 2nd good report from Visiongain and a good price.”
Dr Luz Chapa Azuella, Mexico
American Association of Colleges of Pharmacy
American College of Clinical Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists
American Society of Health-System Pharmacists
Association of Special Pharmaceutical Manufacturers
Australian College of Pharmacy
Biotechnology Industry Organization
Canadian Pharmacists Association
Canadian Society of Hospital Pharmacists
Chinese Pharmaceutical Association
College of Psychiatric and Neurologic Pharmacists
Danish Association of Pharmaconomists
European Association of Employed Community Pharmacists in Europe
European Medicines Agency
Federal Drugs Agency
General Medical Council
Head of Medicines Agency
International Federation of Pharmaceutical Manufacturers & Associations
International Pharmaceutical Federation
International Pharmaceutical Students’ Federation
Medicines and Healthcare Products Regulatory Agency
National Pharmacy Association
Norwegian Pharmacy Association
Ontario Pharmacists Association
Pakistan Pharmacists Association
Pharmaceutical Association of Mauritius
Pharmaceutical Group of the European Union
Pharmaceutical Society of Australia
Pharmaceutical Society of Ireland
Pharmaceutical Society Of New Zealand
Pharmaceutical Society of Northern Ireland
Professional Compounding Centers of America
Royal Pharmaceutical Society
The American Association of Pharmaceutical Scientists
The BioIndustry Association
The Controlled Release Society
The European Federation of Pharmaceutical Industries and Associations
The European Personalised Medicine Association
The Institute of Clinical Research
The International Society for Pharmaceutical Engineering
The Pharmaceutical Association of Israel
The Pharmaceutical Research and Manufacturers of America
The Pharmacy Guild of Australia
The Society of Hospital Pharmacists of Australia
Latest Pharma news
Visiongain Publishes Cell Therapy Technologies Market Report 2024-2034
The cell therapy technologies market is estimated at US$7,041.3 million in 2024 and is projected to grow at a CAGR of 10.7% during the forecast period 2024-2034.
18 April 2024
Visiongain Publishes Automation in Biopharma Industry Market Report 2024-2034
The global Automation in Biopharma Industry market is estimated at US$1,954.3 million in 2024 and is projected to grow at a CAGR of 7% during the forecast period 2024-2034.
17 April 2024
Visiongain Publishes Anti-obesity Drugs Market Report 2024-2034
The global Anti-obesity Drugs market is estimated at US$11,540.2 million in 2024 and is expected to register a CAGR of 21.2% from 2024 to 2034.
12 April 2024
Visiongain Publishes Inflammatory Bowel Diseases (IBD) Drugs Market Report 2024-2034
The global Inflammatory Bowel Diseases (IBD) Drugs market was valued at US$27.53 billion in 2023 and is projected to grow at a CAGR of 6.2% during the forecast period 2024-2034.
11 April 2024


















