The Human Microbiome Market Report 2023-2033: This report will prove invaluable to leading firms striving for new revenue pockets if they wish to better understand the industry and its underlying dynamics. It will be useful for companies that would like to expand into different industries or to expand their existing operations in a new region.
Big Pharma Players Striving To Gain Market Entry Through Collaboration with Microbiome Players
There has been a growing interest from the big pharma players in microbiome research. Many of the big pharma players have been supporting the start-up microbiome players for their clinical drug candidate through funding and collaborations. For instance, in July 2022, Enterome a clinical stage microbiome company signed a strategic R&D collaboration with Nestle Health Science. This license agreement primarily targeted food allergy and Inflammatory Bowel Disease (IBD) under this agreement Enterome will receive an upfront payment of €40 million (US$46.9 million) in cash and equity. In return, Nestle Health Science will be eligible to receive the royalties and sales milestone payments on commercialization of the drug candidates. In February 2021, 4D Pharma Inc. has collaborated with Merck Co Ltd. and Pfizer for its oncology and vaccines to evaluate its MRx5018 in combination with BAVENCIO for treatment of locally advanced or metastatic urothelial carcinoma. Both Merck KGaA and Pfizer Inc. will co-develop and also commercialize it in collaboration. Similarly, in January 2021, Vedanta Biosciences has received US$25 million investment fund from Pfizer as a part of breakthrough growth initiative. Vedanta will use these funds for Phase II study of VE202 indicated for treatment of Inflammatory Bowel Disease. In July 2021, Seres announced its collaboration with Nestle Health Science to co-commercialize SER-109 therapeutic drug for treatment of Clostridioides difficile infection (CDI) in U.S. and Canada. In 2019, Seres had collaborated with AstraZeneca and received US$20 million as a financial support to conduct its research activity for immuno-oncology therapies. With more pharma players are entering this market, the overall market for human microbiome is expected to foresee a staggering growth during the forecast period.
Regulatory Approvals to Challenge Market Growth
The human microbiome has a huge potential in curing and treating diseases that have been difficult to treat through conventional methods. Moreover, the use of Live Biotherapeutic Products (LBPs) has been studied to be of great potential, however lack of defined regulatory approvals for human microbiomes is hampering the growth of this market. There has not been any clearly defined segregation for LBPs and their use, especially when classifying prebiotics, probiotics, and pharmaceutical products. The U.S. FDA in 2010, first time presented draft guidance for microorganisms intended to prevent and treat diseases which got updated in 2016 to officially make a category for LBPs. Similarly, the European Pharmacopeia Commission issued a draft in 2019 with a mention of LBPs to comply with the legislation of biological medicinal products with additional specific guidelines for LBPs. There has been a clear demand from the regulatory authorities regarding the benefits against the risks of LBPs. Conventional GMP practices emphasize the removal of microbes in the final product however, human microbiome products need to be in the final product. Hence, drafting new guidelines for regulatory approvals needs detailed documentation and justification as these products differ from non-therapeutic products. Risk assessment through genomic profiling is crucial as the LBPs have the potential to transfer genes to other microbes or may metabolize other drugs or hormones in the body. To avoid this, in vitro and ex-vivo studies, and animal models studies are essential. Detailed documentation of the origin of the strain, isolation procedure, and cell banking is required. In addition, the difficulty in having consistent batches of LBPs is also a major GMP regulatory hurdle. We believe, that as the market expands there will be well-defined regulatory guidelines for conducting human microbiome studies, at present the approval process poses a major challenge for the market players of this segment.
What Questions Should You Ask before Buying a Market Research Report?
• How is the human microbiome market evolving?
• What is driving and restraining the human microbiome market?
• How will each human microbiome submarket segment grow over the forecast period and how much revenue will these submarkets account for in 2033?
• How will the market shares for each human microbiome submarket develop from 2023 to 2033?
• What will be the main driver for the overall market from 2023 to 2033?
• Will leading human microbiome market broadly follow the macroeconomic dynamics, or will individual national markets outperform others?
• How will the market shares of the national markets change by 2033 and which geographical region will lead the market in 2033?
• Who are the leading players and what are their prospects over the forecast period?
• What are the human microbiome drug pipeline status for these leading companies?
• How will the industry evolve during the period between 2023 and 2033? What are the implications of human microbiome market projects taking place now and over the next 10 years?
• Is there a greater need for product commercialisation to further scale the human microbiome market?
• Where is the human microbiome market heading and how can you ensure you are at the forefront of the market?
• What are the best investment options for new product and service lines?
• What are the key prospects for moving companies into a new growth path and C-suite?
You need to discover how this will impact the human microbiome market today, and over the next 10 years:
• Our 281-page report provides 121 tables and 168 charts/graphs exclusively to you.
• The report highlights key lucrative areas in the industry so you can target them – NOW.
• It contains in-depth analysis of global, regional and national sales and growth.
• It highlights for you the key successful trends, changes and revenue projections made by your competitors.
This report tells you TODAY how the human microbiome market will develop in the next 10 years, and in line with the variations in COVID-19 economic recession and bounce. This market is more critical now than at any point over the last 10 years.
Forecasts to 2033 and other analyses reveal commercial prospects
• In addition to revenue forecasting to 2033, our new study provides you with recent results, growth rates, and market shares.
• You will find original analyses, with business outlooks and developments.
• Discover qualitative analyses (including market dynamics, drivers, opportunities, restraints and challenges), and recent developments.
This report includes data analysis and invaluable insight into how COVID-19 will affect the industry and your company. Four COVID-19 recovery patterns and their impact, namely, “V”, “L”, “W” and “U” are discussed in this report.
Segments Covered in the Report
By Therapeutics
• Gastrointestinal Disorders
• Infectious Diseases
• Metabolic Disorders
• Cancers
• Gut-Brain Axis
• Others
By Type
• Fecal Microbiota Transplant (FMT)
• Live Biotherapeutic Product (LBP)
• Prebiotics
• Post-Biotics
• Precision Antibiotics
By Product
• Prebiotics & Probiotics
• Medicinal Drugs
• Diagnostic Tests
• Skin Microbiome
By Technology
• Genomics
– 16SrRNA Amplicon Sequencing
– Shotgun Metagenomic Sequencing
– Longread Metagenomic Sequencing
• Proteomics
• Metabolomics

In addition to the revenue predictions for the overall world market and segments, you will also find revenue forecasts for five regional and 21 leading national markets:
North America
• U.S.
• Canada
Europe
• Germany
• UK
• France
• Spain
• Italy
• Rest of Europe
Asia Pacific
• China
• Japan
• India
• South Korea
• Taiwan
• Rest of Asia
Latin America
• Brazil
• Mexico
• Rest of Latin America
MEA
• Saudi Arabia
• South Africa
• UAE
• Rest of Middle East & Africa
Need industry data? Please contact us today.
The report also includes profiles and for some of the leading companies in the Human Microbiome Market, 2023 to 2033, with a focus on this segment of these companies’ operations.
Leading companies and the potential for market growth
• 4D Pharma Plc.
• Axial Therapeutics
• BiomX
• Enterobiome
• Enterome
• Finch Therapeutics Group Inc.
• MaaT Pharma
• Rebiotix Inc.,( a Ferring Pharmaceutical Company)
• Second Genome
• Seres Therapeutics
• Synlogic
• Theriva Biologics (ex-Synthetic Biologics)
• Vedanta Biosciences Inc.
Overall world revenue for Human Microbiome Market, 2023 to 2033 in terms of value the market will surpass US$224 million in 2023, our work calculates. We predict strong revenue growth through to 2032. Our work identifies which organizations hold the greatest potential. Discover their capabilities, progress, and commercial prospects, helping you stay ahead.
How will the Human Microbiome Market, 2023 to 2033 report help you?
In summary, our 280+ page report provides you with the following knowledge:
• Revenue forecasts to 2033 for Human Microbiome Market, 2023 to 2033 Market, with forecasts by therapeutics, type, product, technology and company size, each forecast at a global and regional level – discover the industry’s prospects, finding the most lucrative places for investments and revenues.
• Revenue forecasts to 2033 for five regional and 21 key national markets – See forecasts for the Human Microbiome, 2023 to 2033 market in North America, Europe, Asia-Pacific, Latin America, and MEA. Also forecasted is the market in the US, Canada, Mexico, Brazil, Germany, France, UK, Italy, China, India, Japan, and Taiwan among other prominent economies.
• Prospects for established firms and those seeking to enter the market – including company profiles for 13 of the major companies involved in the Human Microbiome Market, 2023 to 2033.
Find quantitative and qualitative analyses with independent predictions. Receive information that only our report contains, staying informed with invaluable business intelligence.
Information found nowhere else
With our new report, you are less likely to fall behind in knowledge or miss out on opportunities. See how our work could benefit your research, analyses, and decisions. Visiongain’s study is for everybody needing commercial analyses for the Human Microbiome Market, 2023 to 2033, market-leading companies. You will find data, trends and predictions.
To access the data contained in this document please email contactus@visiongain.com
Buy our report today Human Microbiome Market Report 2023-2033: Forecasts by Product (Prebiotics, Medical Drugs, Diagnostic Tests, Skin Products), by Type (Fecal Microbiota Transplant, Live Biotherapeutics Products (LBP), Prebiotics, Post-Biotics, Precision Antibiotics), by Technology (Proteomics, Metabolomics, Genomics (16srRNA Sequencing, Shotgun Sequencing and Long Read Metagenomics)), by Application (Gastrointestinal, Infectious Diseases, Metabolic Diseases, Cancers, Gut-Brain Axis, Others ) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Impact and Recovery Pattern Analysis. Avoid missing out by staying informed – order our report now.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for a specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: contactus@visiongain.com
1. Report Overview
1.1 Objectives of the Study
1.2 Introduction to Human Microbiome Market
1.3 What This Report Delivers
1.4 Why You Should Read This Report
1.5 Key Questions Answered by This Analytical Report
1.6 Who is This Report for?
1.7 Methodology
1.7.1 Market Definition
1.7.2 Market Evaluation & Forecasting Methodology
1.7.3 Data Validation
1.7.3.1 Primary Research
1.7.3.2 Secondary Research
1.8 Associated Visiongain Reports
1.9 About Visiongain
1.10 Frequently Asked Questions (FAQs)
2. Executive Summary
3 Market Overview
3.1 Key Findings
3.2 Market Dynamics
3.2.1 Market Driving Factors
3.2.1.1 Wide Array of Therapeutic Applications to Boost Industry Growth
3.2.1.2 Rise in Funding from Private Ventures and Government Institutes to Trigger Industry Growth
3.2.1.3 Big Pharma Players Striving to Gain Market Entry Through Collaboration with Microbiome Players
3.2.1.4 High Throughput Sequencing, Machine Learning (AI & Deep Learning) to Reinforce the Market Growth
3.2.1.5 Microbiome Biomarker Discovery Supporting the Growth of Companion Diagnostics
3.2.2 Market Restraining Factors
3.2.2.1 Regulatory Approvals to Challenge Market Growth
3.2.2.2 Formulation and Translational Manufacturing Obstacles Restraining the Market Growth
3.2.2.3 Lack of Clarity on Pricing and Reimbursement Likely to Hamper Market Growth
3.2.3 Market Opportunities
3.2.3.1 Contract Research Manufacturing Services
3.2.3.2 Nanotechnology to Support Microbiome Diagnostics
3.3 COVID-19 Impact Analysis
3.4 Porter’s Five Forces Analysis
3.4.1 Bargaining Power of Suppliers (Low to Medium)
3.4.2 Bargaining Power of Buyers (Medium to High)
3.4.3 Competitive Rivalry (Medium to High)
3.4.4 Threat from Substitutes (Low to Medium)
3.4.5 Threat of New Entrants (Medium to High)
3.5 PEST Analysis
4 Human Microbiome Market Analysis by Therapeutics
4.1 Key Findings
4.2 Human Microbiome Therapeutics Segment: Market Attractiveness Index
4.3 Human Microbiome Market Size Estimation and Forecast by Therapeutics
4.4 Gastro-Intestinal Disorders
4.4.1 Market Size by Region, 2023-2033 (US$ mn)
4.4.2 Market Share by Region, 2023 & 2033 (%)
4.5 Infectious Diseases
4.5.1 Market Size by Region, 2023-2033 (US$ mn)
4.5.2 Market Share by Region, 2023 & 2033 (%)
4.6 Metabolic Disorder
4.6.1 Market Size by Region, 2023-2033 (US$ mn)
4.6.2 Market Share by Region, 2023 & 2033 (%)
4.7 Cancer
4.7.1 Market Size by Region, 2023-2033 (US$ mn)
4.7.2 Market Share by Region, 2023 & 2033 (%)
4.8 Gut-Brain-Axis
4.8.1 Market Size by Region, 2023-2033 (US$ mn)
4.8.2 Market Share by Region, 2023 & 2033 (%)
4.9 Others
4.9.1 Market Size by Region, 2023-2033 (US$ mn)
4.9.2 Market Share by Region, 2023 & 2033 (%)
5 Human Microbiome Market Analysis by Type
5.1 Key Findings
5.2 Human Microbiome by Type Segment: Market Attractiveness Index
5.3 Human Microbiome Market Size Estimation and Forecast by Therapeutics
5.4 Fecal Microbiota Transplant (FMT)
5.4.1 Market Size by Region, 2023-2033 (US$ mn)
5.4.2 Market Share by Region, 2023 & 2033 (%)
5.5 Live Biotherapeutics Product (LBP)
5.5.1 Market Size by Region, 2023-2033 (US$ mn)
5.5.2 Market Share by Region, 2023 & 2033 (%)
5.6 Prebiotics
5.6.1 Market Size by Region, 2023-2033 (US$ mn)
5.6.2 Market Share by Region, 2023 & 2033 (%)
5.7 Post biotics
5.7.1 Market Size by Region, 2023-2033 (US$ mn)
5.7.2 Market Share by Region, 2023 & 2033 (%)
5.8 Precision Antibiotics
5.8.1 Market Size by Region, 2023-2033 (US$ mn)
5.8.2 Market Share by Region, 2023 & 2033 (%)
6 Human Microbiome Market Analysis by Product
6.1 Key Findings
6.2 Human Microbiome by Product Segment: Market Attractiveness Index
6.3 Human Microbiome Market Size Estimation and Forecast by Product
6.4 Prebiotics & Probiotics
6.4.1 Market Size by Region, 2023-2033 (US$ mn)
6.4.2 Market Share by Region, 2023 & 2033 (%)
6.5 Medicinal Drugs
6.5.1 Market Size by Region, 2023-2033 (US$ mn)
6.5.2 Market Share by Region, 2023 & 2033 (%)
6.6 Diagnostic Tests
6.6.1 Market Size by Region, 2023-2033 (US$ mn)
6.6.2 Market Share by Region, 2023 & 2033 (%)
6.7 Skin Microbiome
6.7.1 Market Size by Region, 2023-2033 (US$ mn)
6.7.2 Market Share by Region, 2023 & 2033 (%)
7 Human Microbiome Market Analysis by Technology
7.1 Key Findings
7.2 Human Microbiome by Technology Segment: Market Attractiveness Index
7.3 Human Microbiome Market Size Estimation and Forecast by Technology
7.4 Genomics
7.4.1 Market Size by Region, 2023-2033 (US$ mn)
7.4.2 Market Share by Region, 2023 & 2033 (%)
7.4.3 Genomics Market by Sequencing Type
7.4.4 16SrRNA Amplicon Sequencing
7.4.5 Shotgun Metagenomic Sequencing
7.4.6 Longread Metagenomic Sequencing
7.5 Proteomics
7.5.1 Market Size by Region, 2023-2033 (US$ mn)
7.5.2 Market Share by Region, 2023 & 2033 (%)
7.6 Metabolomics
7.6.1 Market Size by Region, 2023-2033 (US$ mn)
7.6.2 Market Share by Region, 2023 & 2033 (%)
8 Human Microbiome Market Analysis by Region
8.1 Key Findings
8.2 Regional Market Size Estimation and Forecast
9 North America Human Microbiome Market Analysis
9.1 Key Findings
9.2 North America Human Microbiome Market Attractiveness Index
9.3 North America Human Microbiome Market by Country, 2023, 2028 & 2033 (US$ mn)
9.4 North America Human Microbiome Market Size Estimation and Forecast by Country
9.5 North America Human Microbiome Market Size Estimation and Forecast by Therapeutics
9.6 North America Human Microbiome Market Size Estimation and Forecast by Technology
9.7 North America Human Microbiome Market Size Estimation and Forecast by Type
9.8 North America Human Microbiome Market Size Estimation and Forecast by Product
9.9 U.S. Human Microbiome Market Analysis
9.10 Canada Human Microbiome Market Analysis
10 Europe Human Microbiome Market Analysis
10.1 Key Findings
10.2 Europe Human Microbiome Market Attractiveness Index
10.3 Europe Human Microbiome Market by Country, 2023, 2028 & 2033 (US$ mn)
10.4 Europe Human Microbiome Market Size Estimation and Forecast by Country
10.5 Europe Human Microbiome Market Size Estimation and Forecast by Therapeutics
10.6 Europe Human Microbiome Market Size Estimation and Forecast by Technology
10.7 Europe Human Microbiome Market Size Estimation and Forecast by Type
10.8 Europe Human Microbiome Market Size Estimation and Forecast by Product
10.9 Germany Human Microbiome Market Analysis
10.10 UK Human Microbiome Market Analysis
10.11 France Human Microbiome Market Analysis
10.12 Italy Human Microbiome Market Analysis
10.13 Spain Human Microbiome Market Analysis
10.14 Rest of Europe Human Microbiome Market Analysis
11 Asia-Pacific Human Microbiome Market Analysis
11.1 Key Findings
11.2 Asia-Pacific Human Microbiome Market Attractiveness Index
11.3 Asia-Pacific Human Microbiome Market by Country, 2023, 2028 & 2033 (US$ mn)
11.4 Asia-Pacific Human Microbiome Market Size Estimation and Forecast by Country
11.5 Asia-Pacific Human Microbiome Market Size Estimation and Forecast by Therapeutics
11.6 Asia-Pacific Human Microbiome Market Size Estimation and Forecast by Technology
11.7 Asia-Pacific Human Microbiome Market Size Estimation and Forecast by Type
11.8 Asia-Pacific Human Microbiome Market Size Estimation and Forecast by Product
11.9 China Human Microbiome Market Analysis
11.10 Japan Human Microbiome Market Analysis
11.11 South Korea Human Microbiome Market Analysis
11.12 Taiwan Human Microbiome Market Analysis
11.13 India Human Microbiome Market Analysis
11.14 Rest of Asia Pacific Human Microbiome Market Analysis
12 Latin America Human Microbiome Market Analysis
12.1 Key Findings
12.2 Latin America Human Microbiome Market Attractiveness Index
12.3 Latin America Human Microbiome Market by Country, 2023, 2028 & 2033 (US$ mn)
12.4 Latin America Human Microbiome Market Size Estimation and Forecast by Country
12.5 Latin America Human Microbiome Market Size Estimation and Forecast by Therapeutics
12.6 Latin America Human Microbiome Market Size Estimation and Forecast by Technology
12.7 Latin America Human Microbiome Market Size Estimation and Forecast by Type
12.8 Latin America Human Microbiome Market Size Estimation and Forecast by Product
12.9 Brazil Human Microbiome Market Analysis
12.10 Mexico Human Microbiome Market Analysis
12.11 Rest of Latin America Human Microbiome Market Analysis
13 Middle East & Africa Human Microbiome Market Analysis
13.1 Key Findings
13.2 Middle East & Africa Human Microbiome Market Attractiveness Index
13.3 Middle East & Africa Human Microbiome Market by Country, 2023, 2028 & 2033 (US$ mn)
13.4 Middle East & Africa Human Microbiome Market Size Estimation and Forecast by Country
13.5 Middle East & Africa Human Microbiome Market Size Estimation and Forecast by Therapeutics
13.6 Middle East & Africa Human Microbiome Market Size Estimation and Forecast by Technology
13.7 Middle East & Africa Human Microbiome Market Size Estimation and Forecast by Type
13.8 Middle East & Africa Human Microbiome Market Size Estimation and Forecast by Product
13.9 Saudi Arabia Human Microbiome Market Analysis
13.10 UAE Human Microbiome Market Analysis
13.11 South Africa Human Microbiome Market Analysis
13.12 Rest of Middle East & Africa Human Microbiome Market Analysis
14 Company Profiles
14.1 Company Share Analysis
14.2 Key Business Strategy Analysis
14.3 Rebiotix Inc., (now a Ferring Company)
14.3.1 Company Snapshot
14.3.2 Company Overview
14.3.3 Financial Analysis
14.3.3.1 Net Revenue, 2019-2021
14.3.3.2 R&D, 2019-2021
14.3.4 Rebiotix, Inc.: Pipeline Status
14.3.5 Strategic Outlook
14.4 Seres Therapeutics, Inc.
14.4.1 Company Snapshot
14.4.2 Company Overview
14.4.3 Financial Analysis
14.4.3.1 Revenue, 2017-2021
14.4.3.2 R&D, 2017-2021
14.4.4 Seres Therapeutics, Inc., Pipeline Status
14.4.5 Strategic Outlook
14.5 4D Pharma plc
14.5.1 Company Snapshot
14.5.2 Company Overview
14.5.3 Financial Analysis
14.5.3.1 Revenue, 2017-2021
14.5.3.2 R&D, 2017-2021
14.5.4 4D Pharma plc: Pipeline Status
14.5.5 Strategic Outlook
14.6 Enterobiome
14.6.1 Company Snapshot
14.6.2 Company Overview
14.6.3 Enterobiome: Pipeline Status
14.6.4 Strategic Outlook
14.7 Vedanta Biosciences, Inc.,
14.7.1 Company Snapshot
14.7.2 Company Overview
14.7.3 Financial Analysis
14.7.3.1 Revenue, 2017-2021
14.7.4 Vedanta Biosciences, Inc.: Pipeline Status
14.7.5 Strategic Outlook
14.8 Second Genome
14.8.1 Company Snapshot
14.8.2 Company Overview
14.8.3 Second Genome Inc.: Pipeline Status
14.8.4 Strategic Outlook
14.9 Finch Therapeutics Group Inc.
14.9.1 Company Snapshot
14.9.2 Company Overview
14.9.3 Financial Analysis
14.9.3.1 Revenue, 2020-2021
14.9.3.2 R&D, 2020-2021
14.9.4 Finch Therapeutics Group, Inc.: Pipeline Status
14.9.5 Strategic Outlook
14.10 Enterome
14.10.1 Company Snapshot
14.10.2 Company Overview
14.10.3 Enterome: Pipeline Status
14.10.4 Strategic Outlook
14.11 Theriva Biologics (ex-Synthetic Biologics)
14.11.1 Company Snapshot
14.11.2 Company Overview
14.11.3 Financial Analysis
14.11.3.1 Revenue, 2017-2021
14.11.3.2 R&D, 2017-2021
14.11.4 Theriva Biologics: Pipeline Status
14.11.5 Strategic Outlook
14.12 Synlogic
14.12.1 Company Snapshot
14.12.2 Company Overview
14.12.3 Financial Analysis
14.12.4 Partnerships/ Strategic Collaborations
14.12.5 Strategic Outlook
14.13 Axial Therapeutics
14.13.1 Company Snapshot
14.13.2 Company Overview
14.13.3 Financial Analysis
14.13.4 Axial Therapeutics: Pipeline Status
14.13.5 Strategic Outlook
14.14 MaaT Pharma
14.14.1 Company Snapshot
14.14.2 Company Overview
14.14.3 Financial Analysis
14.14.4 MaaT Pharma: Pipeline Status
14.14.5 Strategic Outlook
14.15 BiomX
14.15.1 Company Snapshot
14.15.2 Company Overview
14.15.3 BiomX: Pipeline Status
14.15.4 Strategic Outlook
15 Conclusion and Recommendations
15.1 Concluding Remarks from Visiongain
15.2 Recommendations for Market Players
List of Tables
Table 1 Human Microbiome Market Snapshot, 2023 & 2033 (US$ million, CAGR %)
Table 2 Human Microbiome Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%)): “U” Shaped Recovery Scenario
Table 3 Human Microbiome Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%)): “W” Shaped Recovery Scenario
Table 4 Human Microbiome Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%)): “L” Shaped Recovery Scenario
Table 5 Human Microbiome Market Forecast by Therapeutics, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 6 Gastrointestinal Disorders Human Microbiome Pipeline Analysis
Table 7 Gastrointestinal Disorders Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%))
Table 8 Infectious Diseases Human Microbiome Pipeline Analysis
Table 9 Infectious Diseases Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%))
Table 10 Metabolic Disorders: Human Microbiome Pipeline Analysis
Table 11 Metabolic Disorders Market Forecast by Region, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 12 Cancer: Human Microbiome Pipeline Analysis
Table 13 Cancer Market Forecast by Region, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 14 Gut-Brain-Axis: Human Microbiome Pipeline Analysis
Table 15 Gut-Brain-Axis Market Forecast by Region, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 16 Others Market Forecast by Region, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 17 Human Microbiome Market Forecast by Type, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 18 Fecal Microbiota Transplant Human Microbiome Pipeline Analysis
Table 19 Fecal Microbiota Transplant Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%))
Table 20 Live Biotherapeutics Product: Pipeline Analysis
Table 21 Live Biotherapeutics Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%))
Table 22 Prebiotics Market Forecast by Region, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 23 Post Biotics: Pipeline Analysis
Table 24 Post Biotics Market Forecast by Region, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 25 Precision Antibiotics Market Forecast by Region, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 26 Human Microbiome Market Forecast by Product, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 27 Prebiotics & Probiotics Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%))
Table 28 Medicinal Drugs Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%))
Table 29 Diagnostic Tests Market Forecast by Region, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 30 Skin Microbiome Market Forecast by Region, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 31 Human Microbiome Market Forecast by Technology, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 32 Genomics Microbiome Technology Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%))
Table 33 Genomics Microbiome Technology Market by Sequencing Types, 2023-2033 (US$ mn, AGR (%), CAGR (%))
Table 34 Proteomics Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%))
Table 35 Metabolomics Market Forecast by Region, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 36 Human Microbiome Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%))
Table 37 North America Human Microbiome Market Forecast by Country, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 38 North America Human Microbiome Market Forecast by Therapeutics, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 39 North America Human Microbiome Market Forecast by Technology, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 40 North America Human Microbiome Market Forecast by Type, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 41 North America Human Microbiome Market Forecast by Product, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 42 U.S. Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 43 Canada Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 44 Europe Human Microbiome Market Forecast by Country, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 45 Europe Human Microbiome Market Forecast by Therapeutics, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 46 Europe Human Microbiome Market Forecast by Technology, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 47 Europe Human Microbiome Market Forecast by Type, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 48 Europe Human Microbiome Market Forecast by Product, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 49 Germany Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 50 U.K. Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 51 France Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 52 Italy Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 53 Spain Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 54 Rest of Europe Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 55 Asia-Pacific Human Microbiome Market Forecast by Country, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 56 Asia-Pacific Human Microbiome Market Forecast by Therapeutics, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 57 Asia-Pacific Human Microbiome Market Forecast by Technology, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 58 Asia-Pacific Human Microbiome Market Forecast by Type, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 59 Asia-Pacific Human Microbiome Market Forecast by Product, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 60 China Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 61 Japan Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 62 South Korea Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 63 Taiwan Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 64 India Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 65 Rest of Asia Pacific Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 66 Latin America Human Microbiome Market Forecast by Country, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 67 Latin America Human Microbiome Market Forecast by Therapeutics, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 68 Latin America Human Microbiome Market Forecast by Technology, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 69 Latin America Human Microbiome Market Forecast by Type, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 70 Latin America Human Microbiome Market Forecast by Product, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 71 Brazil Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 72 Mexico Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 73 Rest of Latin America Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 74 Middle East & Africa Human Microbiome Market Forecast by Country, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 75 Middle East & Africa Human Microbiome Market Forecast by Therapeutics, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 76 Middle East & Africa Human Microbiome Market Forecast by Technology, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 77 Middle East & Africa Human Microbiome Market Forecast by Type, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 78 Middle East & Africa Human Microbiome Market Forecast by Product, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 79 Saudi Arabia Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 80 UAE Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 81 South Africa Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 82 Rest of MEA Human Microbiome Market Forecast, 2023-2033 (US$ mn, AGR%, CAGR %)
Table 83 Key Business Strategies Adopted by Key Players in Human Microbiome Market
Table 84 Rebiotix Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 85 Rebiotix, Inc.: Pipeline Development
Table 86 Rebiotix, Inc.: Strategic Outlook
Table 87 Seres Therapeutics, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 88 Seres Therapeutics, Inc.: Product Pipeline
Table 89 Seres Therapeutics, Inc.: Strategic Outlook
Table 90 4D Pharma plc: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 91 4D Pharma plc: Live Biotherapeutics Pipeline
Table 92 4D Pharma plc: Strategic Outlook
Table 93 Enterobiome: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 94 Enterobiome: Microbiome Pipeline
Table 95 Enterobiome: Strategic Outlook
Table 96 Vedanta Biosciences,Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 97 Vedanta Biosciences (a Puretech Health PLC company),: Pipeline
Table 98 Vedanta Biosciences,Inc.,: Strategic Outlook
Table 99 Second Genome Plc: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 100 Second Genome Inc.: Pipeline Status
Table 101 Second Genome Plc: Strategic Outlook
Table 102 Finch Therapeutics Group, Inc..: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 103 Finch Therapeutics Group, Inc.: Pipeline Status
Table 104 Finch Therapeutics Group, Inc..: Strategic Outlook
Table 105 Enterome: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 106 Enterome: Pipeline Status
Table 107 Enterome: Strategic Outlook
Table 108 Theriva Biologics: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 109 Theriva Biologics: Pipeline Status
Table 110 Theriva Biologics: Strategic Outlook
Table 111 Synlogic: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 112 Synlogic: Pipeline Status
Table 113 Synlogic : Strategic Outlook
Table 114 Axial Therapeutics: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 115 Axial Therapeutics: Pipeline
Table 116 Axial Therapeutics : Strategic Outlook
Table 117 MaaT Pharma: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 118 MaaT Pharma : Pipeline Analysis
Table 119 MaaT : Strategic Outlook
Table 120 BiomX: mRNA Pipeline
Table 121 BiomX: Strategic Outlook
List of Figures
Figure 1 Human Microbiome Market Segmentation
Figure 2 Human Microbiome Market by Technology: Market Attractiveness Index
Figure 3 Human Microbiome Market by Product: Market Attractiveness Index
Figure 4 Human Microbiome Market by Type: Market Attractiveness Index
Figure 5 Human Microbiome Market by Therapeutic Applications: Market Attractiveness Index
Figure 6 Human Microbiome Market Attractiveness Index by Region
Figure 7 Human Microbiome Market: Market Dynamics
Figure 8 Human Microbiome Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%)): “V” Shaped Recovery
Figure 9 Human Microbiome Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%)): “U” Shaped Recovery
Figure 10 Human Microbiome Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%)): “W” Shaped Recovery
Figure 11 Human Microbiome Market by Region, 2023-2033 (US$ mn, AGR (%), CAGR (%)): “L” Shaped Recovery
Figure 12 Human Microbiome: Market: Porter’s Five Forces Analysis
Figure 13 Human Microbiome Market: PEST Analysis
Figure 14 Human Microbiome Market by Therapeutics: Market Attractiveness Index
Figure 15 Human Microbiome Market Forecast by Therapeutics, 2023-2033 (US$ million)
Figure 16 Human Microbiome Market Share Forecast by Therapeutics, 2023, 2028, 2033 (%)
Figure 17 Gastrointestinal Disorders Market Forecast by Region, 2023-2033(US$ million)
Figure 18 Gastrointestinal Disorders Market by Region, 2023 & 2033 (%)
Figure 19 Infectious Diseases Market Forecast by Region, 2023-2033(US$ million)
Figure 20 Infectious Diseases Market by Region, 2023 & 2033 (%)
Figure 21 Metabolic Disorders Market Forecast by Region, 2023-2033 (US$ million)
Figure 22 Metabolic Disorders Market Share Forecast by Region, 2023 & 2033 (%)
Figure 23 Cancer Market Forecast by Region, 2023-2033 (US$ million)
Figure 24 Cancer Market Share Forecast by Region, 2023 & 2033 (%)
Figure 25 Gut-Brain-Axis Market Forecast by Region, 2023-2033 (US$ million)
Figure 26 Gut-Brain-Axis Market Share Forecast by Region, 2023 & 2033 (%)
Figure 27 Others Market Forecast by Region, 2023-2033 (US$ million)
Figure 28 Others Market Share Forecast by Region, 2023 & 2033 (%)
Figure 29 Human Microbiome Market by Type: Market Attractiveness Index
Figure 30 Human Microbiome Market Forecast by Type, 2023-2033 (US$ million)
Figure 31 Human Microbiome Market Share Forecast by Therapeutics, 2023, 2028, 2033 (%)
Figure 32 Fecal Microbiota Transplant Market Forecast by Region, 2023-2033(US$ million)
Figure 33 Fecal Microbiota Transplant Market by Region, 2023 & 2033 (%)
Figure 34 Live Biotherapeutics Product Market Forecast by Region, 2023-2033(US$ million)
Figure 35 Live Biotherapeutics Product Market by Region, 2023 & 2033 (%)
Figure 36 Prebiotics Market Forecast by Region, 2023-2033 (US$ million)
Figure 37 Prebiotics Market Share Forecast by Region, 2023 & 2033 (%)
Figure 38 Post Biotics Market Forecast by Region, 2023-2033 (US$ million)
Figure 39 Post Biotics Market Share Forecast by Region, 2023 & 2033 (%)
Figure 40 Precision Antibiotics Market Forecast by Region, 2023-2033 (US$ million)
Figure 41 Precision Antibiotics Market Share Forecast by Region, 2023 & 2033 (%)
Figure 42 Human Microbiome Market by Type: Market Attractiveness Index
Figure 43 Human Microbiome Market Forecast by Product, 2023-2033 (US$ million)
Figure 44 Human Microbiome Market Share Forecast by Product, 2023, 2028, 2033 (%)
Figure 45 Prebiotics & Probiotics Market Forecast by Region, 2023-2033(US$ million)
Figure 46 Prebiotics & Probiotics Market by Region, 2023 & 2033 (%)
Figure 47 Medicinal Drugs Market Forecast by Region, 2023-2033(US$ million)
Figure 48 Medicinal Drugs Market by Region, 2023 & 2033 (%)
Figure 49 Diagnostics Tests Market Forecast by Region, 2023-2033 (US$ million)
Figure 50 Diagnostics Tests Market Share Forecast by Region, 2023 & 2033 (%)
Figure 51 Skin Microbiome Market Forecast by Region, 2023-2033 (US$ million)
Figure 52 Skin Microbiome Market Share Forecast by Region, 2023 & 2033 (%)
Figure 53 Human Microbiome Market by Technology: Market Attractiveness Index
Figure 54 Human Microbiome Market Forecast by Technology, 2023-2033 (US$ million)
Figure 55 Human Microbiome Market Share Forecast by Therapeutics, 2023, 2028, 2033 (%)
Figure 56 Genomics Microbiome Technology Market Forecast by Region, 2023-2033(US$ million)
Figure 57 Genomics Microbiome Technology Market Forecast by Region, 2023 & 2033 (%)
Figure 58 16sRNA Sequencing Microbiome Technology Market, 2023-2033 (US$ mn, AGR (%),
Figure 59 Shotgun Metagenomic Sequencing Microbiome Technology Market, 2023-2033 (US$ mn, AGR (%),
Figure 60 Longread Metagenomic Sequencing Microbiome Technology Market, 2023-2033 (USD $Mn, AGR (%),
Figure 61 Proteomics Market Forecast by Region, 2023-2033(US$ million)
Figure 62 Proteomics: Market by Region, 2023 & 2033 (%)
Figure 63 Metabolomics Market Forecast by Region, 2023-2033 (US$ million)
Figure 64 Metabolomics Market Share Forecast by Region, 2023 & 2033 (%)
Figure 65 Human Microbiome Market Forecast by Region 2023, 2033 (Revenue, CAGR %)
Figure 66 Human Microbiome Market Share Forecast by Region 2023, 2028, 2033 (%)
Figure 67 Human Microbiome Market by Region, 2023-2033 (US$ mn)
Figure 68 North America Human Microbiome Market Attractiveness Index
Figure 69 North America Human Microbiome Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 70 North America Human Microbiome Market Forecast by Country, 2023-2033 (US$ million)
Figure 71 North America Human Microbiome Market Share Forecast by Country, 2023 & 2033 (%)
Figure 72 North America Human Microbiome Market Forecast by Therapeutics, 2023-2033 (US$ million)
Figure 73 North America Human Microbiome Market Share Forecast by Therapeutics, 2023 & 2033 (%)
Figure 74 North America Human Microbiome Market Forecast by Technology, 2023-2033 (US$ million)
Figure 75 North America Human Microbiome Market Share Forecast by Technology, 2023 & 2033 (%)
Figure 76 North America Human Microbiome Market Forecast by Type, 2023-2033 (US$ million)
Figure 77 North America Human Microbiome Market Share Forecast by Type, 2023 & 2033 (%)
Figure 78 North America Human Microbiome Market Forecast by Product, 2023-2033 (US$ million)
Figure 79 North America Human Microbiome Market Share Forecast by Product, 2023 & 2033 (%)
Figure 80 U.S. Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 81 Canada Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 82 Europe Human Microbiome Market Attractiveness Index
Figure 83 Europe Human Microbiome Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 84 Europe Human Microbiome Market Forecast by Country, 2023-2033 (US$ million)
Figure 85 Europe Human Microbiome Market Share Forecast by Country, 2023 & 2033 (%)
Figure 86 Europe Human Microbiome Market Forecast by Therapeutics, 2023-2033 (US$ million)
Figure 87 Europe Human Microbiome Market Share Forecast by Therapeutics, 2023 & 2033 (%)
Figure 88 Europe Human Microbiome Market Forecast by Technology, 2023-2033 (US$ million)
Figure 89 Europe Human Microbiome Market Share Forecast by Technology, 2023 & 2033 (%)
Figure 90 Europe Human Microbiome Market Forecast by Type, 2023-2033 (US$ million)
Figure 91 Europe Human Microbiome Market Share Forecast by Type, 2023 & 2033 (%)
Figure 92 Europe Human Microbiome Market Forecast by Product, 2023-2033 (US$ million)
Figure 93 Europe Human Microbiome Market Share Forecast by Product, 2023 & 2033 (%)
Figure 94 Germany Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 95 U.K. Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 96 France Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 97 Italy Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 98 Spain Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 99 Rest of Europe Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 100 Asia-Pacific Human Microbiome Market Attractiveness Index
Figure 101 Asia-Pacific Human Microbiome Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 102 Asia-Pacific Human Microbiome Market Forecast by Country, 2023-2033 (US$ million)
Figure 103 Asia-Pacific Human Microbiome Market Share Forecast by Country, 2023 & 2033 (%)
Figure 104 Asia-Pacific Human Microbiome Market Forecast by Therapeutics, 2023-2033 (US$ million)
Figure 105 Asia-Pacific Human Microbiome Market Share Forecast by Therapeutics, 2023 & 2033 (%)
Figure 106 Asia-Pacific Human Microbiome Market Forecast by Technology, 2023-2033 (US$ million)
Figure 107 Asia-Pacific Human Microbiome Market Share Forecast by Technology, 2023 & 2033 (%)
Figure 108 Asia-Pacific Human Microbiome Market Forecast by Type, 2023-2033 (US$ million)
Figure 109 Asia-Pacific Human Microbiome Market Share Forecast by Type, 2023 & 2033 (%)
Figure 110 Asia-Pacific Human Microbiome Market Forecast by Product, 2023-2033 (US$ million)
Figure 111 Asia-Pacific Human Microbiome Market Share Forecast by Product, 2023 & 2033 (%)
Figure 112 China Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 113 Japan Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 114 South Korea Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 115 Taiwan Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 116 India Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 117 Rest of Asia Pacific Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 118 Latin America Human Microbiome Market Attractiveness Index
Figure 119 Latin America Human Microbiome Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 120 Latin America Human Microbiome Market Forecast by Country, 2023-2033 (US$ million)
Figure 121 Latin America Human Microbiome Market Share Forecast by Country, 2023 & 2033 (%)
Figure 122 Latin America Human Microbiome Market Forecast by Therapeutics, 2023-2033 (US$ million)
Figure 123 Latin America Human Microbiome Market Share Forecast by Therapeutics, 2023 & 2033 (%)
Figure 124 Latin America Human Microbiome Market Forecast by Technology, 2023-2033 (US$ million)
Figure 125 Latin America Human Microbiome Market Share Forecast by Technology, 2023 & 2033 (%)
Figure 126 Latin America Human Microbiome Market Forecast by Type, 2023-2033 (US$ million)
Figure 127 Latin America Human Microbiome Market Share Forecast by Type, 2023 & 2033 (%)
Figure 128 Latin America Human Microbiome Market Forecast by Product, 2023-2033 (US$ million)
Figure 129 Latin America Human Microbiome Market Share Forecast by Product, 2023 & 2033 (%)
Figure 130 Brazil Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 131 Mexico Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 132 Rest of Latin America Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 133 Middle East & Africa Human Microbiome Market Attractiveness Index
Figure 134 Middle East & Africa Human Microbiome Market by Region, 2023, 2028 & 2033 (US$ million)
Figure 135 Middle East & Africa Human Microbiome Market Forecast by Country, 2023-2033 (US$ million)
Figure 136 Middle East & Africa Human Microbiome Market Share Forecast by Country, 2023 & 2033 (%)
Figure 137 Middle East & Africa Human Microbiome Market Forecast by Therapeutics, 2023-2033 (US$ million)
Figure 138 Middle East & Africa Human Microbiome Market Share Forecast by Therapeutics, 2023 & 2033 (%)
Figure 139 Middle East & Africa Human Microbiome Market Forecast by Technology, 2023-2033 (US$ million)
Figure 140 Middle East & Africa Human Microbiome Market Share Forecast by Technology, 2023 & 2033 (%)
Figure 141 Middle East & Africa Human Microbiome Market Forecast by Type, 2023-2033 (US$ million)
Figure 142 Middle East & Africa Human Microbiome Market Share Forecast by Type, 2023 & 2033 (%)
Figure 143 Middle East & Africa Human Microbiome Market Forecast by Product, 2023-2033 (US$ million)
Figure 144 Middle East & Africa Human Microbiome Market Share Forecast by Product, 2023 & 2033 (%)
Figure 145 Saudi Arabia Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 146 UAE Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
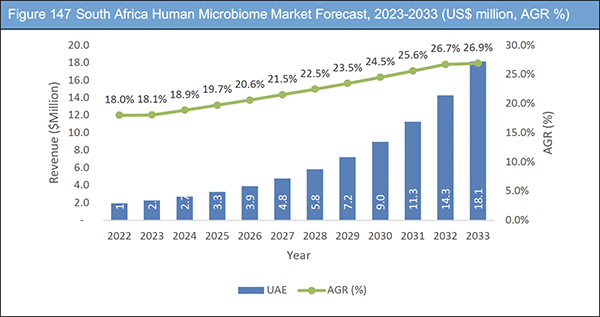
Figure 147 South Africa Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 148 Rest of MEA Human Microbiome Market Forecast, 2023-2033 (US$ million, AGR %)
Figure 149 Rebiotix, Inc.: Net Revenue, 2019-2021 (US$ million)
Figure 150 Rebiotix, Inc.: R&D, 2019-2021 (US$ million)
Figure 151 Seres Therapeutics, Inc.: Revenue, 2017-2021 (US$ million)
Figure 152 Seres Therapeutics, Inc.: R&D, 2017-2021 (US$ million)
Figure 153 4D Pharma plc: Revenue, 2017-2021 (US$ million)
Figure 154 4D Pharma plc: R&D Expenses, 2017-2021 (US$ million)
Figure 155 Vedanta Biosciences (a Puretech Health PLC company): Revenue, 2017-2021 (US$ million)
Figure 156 Finch Therapeutics Group, Inc.: Revenue, 2020-2021 (US$ million)
Figure 157 Finch Therapeutics Group, Inc.: R&D Expenses, 2020-2021 (US$ million)
Figure 158 Theriva Biologics: Revenue, 2017-2021 (US$ million)
Figure 159 Theriva Biologics: R&D 2017-2021 (US$ million)
Figure 160 Synlogic Inc. Revenues: 2017-2021 (US$ million)
Figure 161 Synlogic Inc. R&D Expenses 2017-2021 (US$ million)
List of Companies Profiled in the report
4D Pharma Plc.
Axial Therapeutics
BiomX
Enterobiome
Enterome
Finch Therapeutics Group Inc.
MaaT Pharma
Rebiotix Inc.,( a Ferring Pharmacetical Company)
Second Genome
Seres Therapeutics
Synlogic
Theriva Biologics (ex-Synthetic Biologics)
Vedanta Biosciences Inc.
List of Other Notable Players
Adaptive Phage Therapeutics
ADM
AsiaBiome
Bacthera
Beiersdorf
Boehringer Ingelheim
Holobiome
Infant Bacterial Therapeutics
Microba Life Sciences
Microbiotica
NovoBiome
NuBiyota
Osel
Sensei Therapeutics
Xbiome
List of Organizations Mentioned in the Report
Biomedical Advanced Research and Development Authority (BARDA)
California Institute of Technology
Canadian Institute of Health Research (CHIR)
Centro para el Desarrollo Tecnológico Industrial, Spain
Food and Drug Administration (US FDA)
France’s National Research Institute for Agriculture, Food and Environment (INRAE)
German Research Foundation
India Ministry of Science and Technology
Instituto Nacional de Medicina Genómica (INMEGEN)
Jansen Microbiome Research Center
Madrid Science Park and National Center for Genomic Analysis (CNAG)
National Committee for Biosafety, Biosecurity and Life Sciences (CNBBSV)
National Institute of Agricultural Research (INIA)
National Institute of Health (NIH)
National Institutes of Science and Technology (INCT), Brazil
Sau Paulo Research Foundation (FAPESP)
The African Microbiome Institute (AMI)
The Biological Sciences Research Council (BBSRC)
The Combating Antibiotic Resistant Bacteria (CARB-X)
The Japan Microbiome Consortium




















