
The new report from business intelligence provider Visiongain offers an updated outlook for the global clinical research organizations (CROs) market. Visiongain assesses that the clinical research organizations (CROs) market will generate revenues of $38.0 billion in 2020. Contract research organizations (CROs) have become essential to pharmaceutical, biotech and other medical-related industries by supporting their clients’ efforts to test, refine and market the latest pharmaceuticals and medical devices through clinical trials.
The global CRO market value has grown by nearly a 9.5% compound annual growth rate (CAGR) over the past several years and is expected to reach $38 billion in 2020 and $94 billion by 2030, as specialized demand, patent expiration and proliferation of generic medications are all leading to greater outsourcing of work to CROs. Also driving the trend toward CRO outsourcing is pharmaceutical companies’ (pharma companies’) increasing need to reduce clinical trial costs and improve efficiencies. This in turn is increasing time-to-market urgencies for pharma companies while also leading to pricing pressures and greater margin awareness. Pharmaceutical companies are focusing on niche sector CROs to expand their knowledgebase in highly complex trial areas, and functional service provider (FSP) contracts are allowing them the ability to outsource individual services rather than entire studies or projects. This integrated Pharma-CRO partnership leads to efficiency gains and is often desirable for studies of high complexity, tight time constraints and/or broader scope.
Strong demand and increased competitiveness are facilitating mergers and partnerships to enhance larger companies’ full-service capabilities and international reach. Recovery of capital markets and strong revenue potential are boosting CRO valuations, which is also increasing merger and acquisition (M&A) activity and public offerings in the industry.
CROs are benefiting from top-line growth among deep-pocketed pharmaceutical companies in addition to several drivers specific to current industry trends. Pharmaceutical companies are bringing a record number of drugs to market in a more time-sensitive and competitive environment. As acceleration of time-to-market and increased cost-effectiveness have become critical, CROs are capitalizing on their expertise in specific areas of the clinical research process.
Pharma pipelines are currently ripe with new-age, complex therapies that are nearing market readiness. Regenerative cell-based therapies, chimeric antigen receptor (CAR)-modified T cell therapy (CAR-T), immune-oncology combinations and innovative autoimmune therapies are just some of the new fields being studied in clinical trials across the globe. More adept use of data and analytics by pharma and CROs is leading to more appropriate patient trial selection and more adaptive trial designs. Adaptive design studies, risk-based monitoring and other flexible approaches can reduce costs while allowing adaptions as studies progress. These trends mitigate risks for pharma companies and patient pools while facilitating the pursuit of more potential therapies and enhancing the drug discovery pipeline. Correspondingly, patient receptiveness is at an all-time high. These factors are all contributing to the rapid proliferation of clinical trials in the U.S. and throughout the globe.
How this report will benefit you
Read on to discover how you can exploit the future business opportunities emerging in the clinical research organizations (CROs) sector. Visiongain’s new study tells you and tells you NOW.
In this brand-new report, you will receive 214 in-depth tables, charts and graphs– all unavailable elsewhere.
The 215-page report provides clear detailed insight into the global clinical research organizations (CROs) market. It reveals the key drivers and challenges affecting the market.
By ordering and reading our brand-new report today you will be better informed and ready to act.
Report Scope
• Global clinical research organizations (CROs) market forecasts from 2020-2030
• Regional clinical research organizations (CROs) market forecasts from 2020-2030 covering
– North America
– South America
– Western Europe
– Eastern Europe
– Asia-Pacific
– Middle East and Africa
• Country level clinical research organizations (CROs) forecasts from 2020-2030 covering
– US
– Canada
– Mexico
– UK
– Germany
– Japan
– Brazil
– India
– RoW
• Clinical Research Organizations (CROs) submarket forecasts from 2020-2030 covering services
– Drug Discovery
– Pre-Clinical & Clinical
– Laboratory Services
– Other Service
• Clinical Research Organizations (CROs) submarket forecasts from 2020-2030 covering Application
– Cardiology
– Infectious Disease
– Oncology
– Metabolic Disorders
– Other Applications
• Clinical Research Organizations (CROs) submarket forecasts from 2020-2030 covering End-Users
– Pharmaceutical & Biotechnological Companies
– Academic & Research Institutes
• Analysis of the key factors driving growth in the global, regional and country level clinical research organizations (CROs) markets from 2020-2030
Profiles and competitive positioning map of the leading 10 clinical research organizations (CROs) companies
• Charles River Laboratories
• Frontage Laboratories, Inc.,
• Icon Plc
• Syneos Health, Inc. (Inc Research/Inventive Health)
• LabCorp/Covance
• Parexel
• Pharmaceutical Product Development
• PRA Health Services
• Quintiles
• WuXiAppTec
How will you benefit from this report?
• This report will keep your clinical research organizations (CROs) knowledge base up to speed. Don’t get left behind
• This report will reinforce strategic decision decision-making based upon definitive and reliable market data
• You will learn how to exploit new clinical research organizations (CROs) technological trends
• You will be able to realise your company’s full potential within the market
• You will better understand the competitive landscape and identify potential new business opportunities & partnerships
Who should read this report?
• Anyone within the pharma & value chain
• Investment analysts
• Investment consultancies
• CEO’s
• COO’s
• CIO’s
• Business development managers
• Marketing managers
Visiongain’s study is intended for anyone requiring commercial analyses for the clinical research organizations (CROs) market and leading companies. You will find data, trends and predictions.
Buy our report today Global Contract Research Organisations (CROs) Market: Forecasts & Analysis by Service, by Application, by End-User Including Forecasts by Major Regions and Countries, Plus Profiles of Leading Companies in the Market. Avoid missing out by staying informed – order our report now.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: sara.peerun@visiongain.com
1. REPORT OVERVIEW
1.1 GLOBAL CONTRACT RESEARCH ORGANIZATIONS (CRO) MARKET OVERVIEW
1.1.1 Pharma-CRO Relationships
1.2 GLOBAL CONTRACT RESEARCH ORGANIZATIONS (CRO) MARKET SEGMENTATION
1.3 MARKET DEFINITION
1.4 HOW THIS REPORT DELIVERS
1.5 KEY QUESTIONS ANSWERED BY THIS ANALYTICAL REPORT INCLUDE:
1.6 WHY YOU SHOULD READ THIS REPORT?
1.7 WHO IS THIS REPORT FOR?
1.8 METHODOLOGY
1.9 FREQUENTLY ASKED QUESTIONS (FAQ)
1.10 ASSOCIATED VISIONGAIN REPORTS
1.11 ABOUT VISIONGAIN
2. GLOBAL CONTRACT RESEARCH ORGANIZATIONS (CRO) MARKET
2.1. GLOBAL CONTRACT RESEARCH ORGANIZATIONS MARKET SIZE AND FORECAST (2020-2030)
2.2. GLOBAL CONTRACT RESEARCH ORGANIZATIONS MARKET BY SERVICE
2.3. GLOBAL CONTRACT RESEARCH ORGANIZATIONS MARKET BY APPLICATION
2.3.1 Oncology
2.3.2 Infectious Diseases
2.3.3 Metabolic Disorders
2.3.4 Cardiology
2.4. GLOBAL CONTRACT RESEARCH ORGANIZATIONS MARKET BY END-USER
2.4.1 PHARMACEUTICAL & BIOTECHNOLOGICAL COMPANIES
2.4.2 ACADEMIC RESEARCH ORGANIZATION (ARO)
2.5. GLOBAL CONTRACT RESEARCH ORGANIZATIONS MARKET BY REGION
3. CLINICAL TRIAL PROCESS AND CRO FUNCTIONAL SERVICES-AN OVERVIEW
3.1 INTRODUCTION
3.2 CLINICAL TRIAL PROCESS AND STAGES
3.2.1 Pre-discovery
3.2.2 Drug Discovery
3.2.3 Preclinical
3.2.4 Clinical
3.3 PHASE I
3.4 PHASE II
3.5 PHASE III
3.6 POST-MARKETING SURVEILLANCE AND PHASE IV STUDIES
3.7 REGULATORY ENVIRONMENT
3.7.1 FDA REGULATION AND IMPACT
3.7.2 The Declaration of Helsinki
3.7.3 CDISC Standards
4. GLOBAL CONTRACT RESEARCH ORGANIZATIONS MARKET TRENDS
4.1 INCREASING COMPLEXITY OF CLINICAL TRIALS
4.2 SMALL AND MID-SIZED BIOPHARMA INNOVATION
4.2.1 Small Molecule Versus Biologics
4.3 BIOSIMILARS
4.4 ADAPTIVE TRIAL DESIGN
4.5 RISK-BASED MONITORING
4.6 REAL WORLD EVIDENCE
4.7 REPURPOSING EXISTING DRUGS FOR NEW INDICATIONS
4.8 INCREASED COMPETITION THROUGH THE RESURGENCE OF THE GENERIC DRUG MARKET
4.9 SWOT ANALYSIS OF GLOBAL CLINICAL RESEARCH ORGANIZATIONS
5. NORTH AMERICA MARKET FOR CONTRACT RESEARCH ORGANIZATION
5.1 NORTH AMERICA CRO MARKET BY SERVICE
5.2 NORTH AMERICA CRO MARKET BY APPLICATION
5.3 NORTH AMERICA CRO MARKET BY END USERS
5.4 NORTH AMERICA CRO MARKET BY, COUNTRY
5.4.1 United States of America
5.4.2 Canada
5.4.3 Mexico
6. EUROPE MARKET FOR CONTRACT RESEARCH ORGANISATION
6.1 EUROPE CRO MARKET BY SERVICE
6.2 EUROPE CRO MARKET BY APPLICATIONS
6.3 EUROPE CRO BY END USERS
6.4 EUROPE CRO BY COUNTRY
6.4.1 Germany
6.4.2 United Kingdom
6.4.3 France
6.4.4 Spain
6.4.5 Italy
7. ASIA-PACIFIC MARKET FOR CONTRACT RESEARCH ORGANISATION
7.1 ASIA-PACIFIC CRO MARKET BY SERVICE
7.2 ASIA-PACIFIC CRO BY APPLICATION
7.3 ASIA-PACIFIC CRO BY END USER
7.4 ASIA-PACIFIC CRO MARKET BY COUNTRY
7.4.1 China
7.4.2 India
7.4.3 Japan
7.4.4 Australia
8. REST OF WORLD MARKET FOR CONTRACT RESEARCH ORGANISATION
8.1 REST OF WORLD CRO BY SERVICE
8.2 REST OF WORLD CRO BY APPLICATIONS
8.3 REST OF WORLD CRO BY END USERS
8.4 REST OF WORLD CRO BY COUNTRY
8.4.1 South Africa
8.4.2 Brazil
9. COMPETITIVE LANDSCAPE
9.1 FUNCTIONAL SERVICE PROVIDER RELATIONSHIPS
9.2 OFF SHORING
9.3 THERAPEUTIC AREAS IN DRUG DEVELOPMENT
9.4 CURRENT PHARMA-CRO CONTRACTS
9.5 LARGEST THERAPEUTIC AREAS RELY ON KEY CRO EXPERTISE
9.6 MERGERS AND ACQUISITIONS
9.6.1 Recent Merger and Acquisition Activity
9.6.1.1 Charles River Laboratories
10 COMPANY PROFILES
10.1 CHARLES RIVER LABORATORIES
10.1.1 Company Overview
10.1.2 Company Financials
10.1.3 Company Recent Developments
10.2 FRONTAGE LABORATORIES, INC.,
10.2.1 Company Overview
10.2.2 Company Financials
10.2.3 Company Recent Developments
10.3 ICON PLC
10.3.1 Company Overview
10.3.2 Company Products & Services
10.3.3 Company Financials
10.3.4 Company Recent Developments
10.4 SYNEOS HEALTH, INC. (INC RESEARCH/INVENTIVE HEALTH)
10.4.1 Company Overview
10.4.2 Company Financials
10.4.3 Company Recent Developments
10.5 LABCORP/COVANCE
10.5.1 Company Overview
10.5.2 Company Financials
10.5.3 Company Recent Developments
10.6PAREXEL
10.6.1 Company Overview
10.6.2 Company Products & Services
10.6.3 Company Financials
10.7 PHARMACEUTICAL PRODUCT DEVELOPMENT
10.7.1 Company Overview
10.7.2 Company Products & Services
10.7.3 Company Recent Developments
10.8 PRA HEALTH SERVICES
10.8.1 Company Overview
10.8.2 Company Products & Services
10.8.3 Company Financials
10.8.4 Company Recent Developments
10.9 QUINTILES
10.9.1 Company Overview
10.9.2 Company Financials
10.9.3 Company Recent Developments
10.10 WUXIAPPTEC
10.10.1 Company Overview
10.10.2 Company Products & Services
10.10.3 Company Financials
10.10.4 Company Recent Developments
11.CONCLUSION
12. GLOSSARY
12.1 TECHNICAL DEFINITIONS
ASSOCIATED VISIONGAIN REPORTS
VISIONGAIN REPORT SALES ORDER FORM
APPENDIX A
ABOUT VISIONGAIN
APPENDIX B
VISIONGAIN REPORT EVALUATION FORM
List of Tables
Table 2.1 Global Contract Research Organizations Market, 2020-2030 (US$ Billion)
Table 2.2 Global Contract Research Organizations Market by Service, 2020-2030 (US$ Billion)
Table 2.3 Global Contract Research Organizations Market by End-Use, 2020-2030 (US$ Billion)
Table 2.4 Global Contract Research Organizations Market by Application, 2020-2030 (US$ Billion)
Table 2.5 Global Contract Research Organizations Market by Region, 2020-2030 (US$ Billion)
Table 3.1 Likelihood of Approval, by Major Disease Area/Phase (%)
Table 3.2 Clinical Phase Description
Table 3.3 New Drug Approvals: New Drugs Entering the Market at an Increasing Pace Until Last Year, 2006–2018
Table 5.1 North America Contract Research Organizations Market by Service, 2020-2030 (US$ Billion)
Table 5.2 North America Contract Research Organizations Market by End-Use, 2020-2030 (US$ Billion)
Table 5.3 North America Contract Research Organizations Market by Application, 2020-2030 (US$ Billion)
Table 5.4 North America Contract Research Organizations Market by Country, 2020-2030 (US$ Billion)
Table 5.5 List of Contract Research Organizations in U.S.
Table 5.6 List of Contract Research Organizations in Canada
Table 5.7 List of Contract Research Organizations in Mexico
Table 6.1 Europe Contract Research Organizations Market by Service, 2020-2030 (US$ Billion)
Table 6.2 Europe Contract Research Organizations Market by End-Use, 2020-2030 (US$ Billion)
Table 6.3 Europe Contract Research Organizations Market by Application, 2020-2030 (US$ Billion)
Table 6.4 Europe Contract Research Organizations Market by Country, 2020-2030 (US$ Billion)
Table 6.5 List of Contract Research Organizations in Germany
Table 6.6 List of Contract Research Organizations in the U.K.
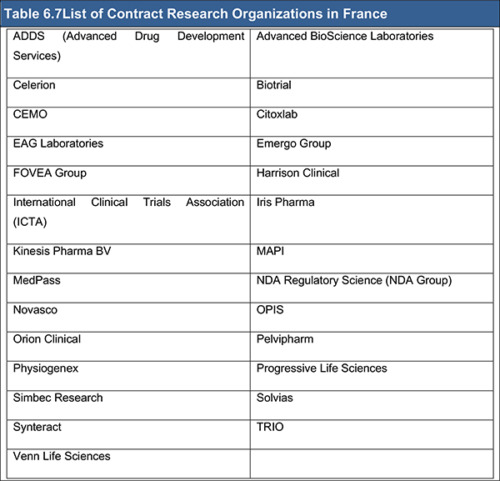
Table 6.7 List of Contract Research Organizations in France
Table 6.8 List of Contract Research Organizations in Spain
Table 6.9 List of Contract Research Organizations in Italy
Table 7.1 Asia-Pacific Contract Research Organizations Market by Service, 2020-2030 (US$ Billion)
Table 7.2 Asia-Pacific Contract Research Organizations Market by End-Use, 2020-2030 (US$ Billion)
Table 7.3 Asia-Pacific Contract Research Organizations Market by Application, 2020-2030 (US$ Billion)
Table 7.4 Asia-Pacific Contract Research Organizations Market by Country, 2020-2030 (US$ Billion)
Table 7.5 List of Contract Research Organizations in China
Table 7.6 List of Contract Research Organizations in India
Table 7.7 List of Contract Research Organizations in Japan
Table 7.8 List of Contract Research Organizations in Australia
Table 7.9 RoW Contract Research Organizations Market by Service, 2020-2030 (US$ Billion)
Table 7.10 RoW Contract Research Organizations Market by End-Use, 2020-2030 (US$ Billion)
Table 8.1 RoW Contract Research Organizations Market by Application, 2020-2030 (US$ Billion)
Table 8.2 RoW Contract Research Organizations Market by Country, 2020-2030 (US$ Billion)
Table 8.3 List of Contract Research Organizations in South Africa
Table 8.4 List of Contract Research Organizations in Brazil
Table 9.1 Comparative View of Services Provided by CRO Companies
Table 9.2 Key Players in Therapeutic Areas
Table 9.3 Strategic alliances, CROs and pharma/CRO alliances are shaping future landscape.
Table 9.4 M &A Activity Among Pharma/CROs: Recent M &A Deals in Pharma Outsourcing Sector ($ Millions)
Table 9.5 Deal Types in CRO Sector: Improving Capital Markets Leading to More IPOs ($ Millions)
Table 10.1 Charles River Laboratories Company Details
Table 10.2 Charles River Laboratories Product and Service Offerings
Table 10.3 Charles River Laboratories Recent Developments
Table 10.4 Frontage Laboratories, Inc., Company Details
Table 10.5 Frontage Laboratories, Inc., Services
Table 10.6 Frontage Laboratories, Inc., Company Recent Developments
Table 10.7 Icon Plc Company Details
Table 10.8 ICON Product and Service Offerings
Table 10.9 ICON Company Recent Developments
Table 10.10 Syneos Health, Inc. Company Details
Table 10.11 Syneos Health, Inc. Company Recent Developments
Table 10.12 LabCorp/Covance Company Details
Table 10.13 LabCorp/Covance Company Recent Developments
Table 10.14 Parexel Company Details
Table 10.15 Parexel Product and Service Offerings
Table 10.16 Pharmaceutical Product Development Company Details
Table 10.17 Pharmaceutical Product Development Product and Service Offerings
Table 10.18 Pharmaceutical Product Development Company Recent Developments
Table 10.19 PRA Health Services Company Details
Table 10.20 PRA Health Services Product and Service Offerings
Table 10.21 PRA Health Services Company Recent Developments
Table 10.22 Quintiles Company Details
Table 10.23 Quintiles Product and Service Offerings
Table 10.24 WuXiAppTec Company Details
Table 10.25 WuXiAppTec Product and Service Offerings
Table 10.26 WuXiAppTec Company Recent Developments
List of Figures
Figure 1.1 Total Number of Registered Clinical Studies Worldwide, 2000-2019
Figure 1.2 Global Contract Research Organizations (CRO) Market Segmentation
Figure 2.1 Global Contract Research Organizations Market, 2020-2030 (US$ Billion)
Figure 2.2 Global Contract Research Organizations Market by Service, 2020-2030 (US$ Billion)
Figure 2.3 Global Contract Research Organizations Market by End-Use, 2020-2030 (US$ Billion)
Figure 2.4 Global Contract Research Organizations Market by Application, 2020-2030 (US$ Billion)
Figure 2.5 Global Contract Research Organizations Market by Region, 2020-2030 (US$ Billion)
Figure 3.1 Stages of Clinical Developments
Figure 3.2 Drug Approval Timeline
Figure 4.1 Total Number of Trial Registrations by WHO, Region, 1999-2018
Figure 5.1 Contract Research Organization Map in U.S. ( 8887 Labs)
Figure 5.2 Contract Research Organization Map in Canada (663 Labs)
Figure 5.3 Contract Research Organization Map in Mexico (29 Labs)
Figure 6.1 Contract Research Organization Map in Germany ( 1050 Labs)
Figure 6.2 Contract Research Organization Map in the U.K. ( 1378 Labs)
Figure 6.3 Contract Research Organization Map in France ( 534 Labs)
Figure 6.4 Contract Research Organization Map in Spain (237 Labs)
Figure 6.5 Contract Research Organization Map in Italy (284 Labs)
Figure 7.1 Contract Research Organization Map in China (653 Labs)
Figure 7.2 Contract Research Organization Map in India (589 Labs)
Figure 7.3 Contract Research Organization Map in Japan (279 Labs)
Figure 7.4 Contract Research Organization Map in Australia (254 Labs)
Figure 8.1 Contract Research Organization Map in South Africa ( 53 Labs)
Figure 8.2 Contract Research Organization Map in Brazil (45 Labs)
Figure 9.1 Global CRO Competitor Market Share, by Company, 2019
Figure 9.2 Leading CROs’ Preclinical CRO Market Share, by Company, 2019
Figure 10.1 Charles River Laboratories Total Revenue 2016-2018
Figure 10.2 Charles River Laboratories EBITA 2016-2018
Figure 10.3 Charles River Laboratories Net Income 2016-2018
Figure 10.4 Frontage Laboratories, Inc. Total Revenue 2016-2018
Figure 10.5 Frontage Laboratories, Inc. EBITA 2016-2018
Figure 10.6 Frontage Laboratories, Inc. Net Income 2016-2018
Figure 10.7 ICON Company Total Revenue 2016-2018
Figure 10.8 ICON Company Total EBITA 2016-2018
Figure 10.9 ICON Company Net Income 2016-2018
Figure 10.10 Syneos Health, Inc. Total Revenue 2016-2018
Figure 10.11 Syneos Health, Inc. EBITA 2016-2018
Figure 10.12 Syneos Health, Inc. Net Income 2016-2018
Figure 10.13 LabCorp/Covance Company Total Revenue 2016-2018
Figure 10.14 LabCorp/Covance Company EBITA 2016-2018
Figure 10.15 LabCorp/Covance Company Net Income 2016-2018
Figure 10.16 Parexel Company Total Revenue 2016-2018
Figure 10.17 Parexel Company EBITA 2016-2018
Figure 10.18 Parexel Company Net Income 2016-2018
Figure 10.19 PRA Health Services Company Total Revenue 2016-2018
Figure 10.20 PRA Health Services Company EBITA 2016-2018
Figure 10.21 PRA Health Services Company Net Income 2016-2018
Figure 10.22 Quintiles Company Total Revenue 2016-2018
Figure 10.23 Quintiles Company EBITA 2016-2018
Figure 10.24 Quintiles Company Net Income 2016-2018
Table 10.25 Quintiles Company Recent Developments
Figure 10.26 WuXiAppTec Total Revenue 2016-2018
Figure 10.27 WuXiAppTec EBITA 2016-2018
Figure 10.28 WuXiAppTec Net Income 2016-2018
Companies Profiles
Charles River Laboratories
Frontage Laboratories, Inc.,
Icon Plc
Syneos Health, Inc. (Inc Research/Inventive Health)
LabCorp/Covance
Parexel
Pharmaceutical Product Development
PRA Health Services
Quintiles
WuXiAppTec
Other Companies
4Clinics
Aagami
Absorption Systems
Accelsiors
ACI Clinical
Actimus Bio
Advanced BioScience Laboratories
Advanced Clinical Research Institute
Agilent
Albuquerque Clinical Trials (ACT)
AlcheraBio
Allphase Clinical Research
Amarex Clinical Research
APCER Life Sciences
Applied Healthcare Resource Management (AHRM)
ArisGloba
At Clinical Systems
Avail Clinical Research
Axio Research
AXON
Universal Research Group
BBK
Bio Reliance Corporation
BioFortis
BioRASI Clinical Research
Biotrial
Boston MedTech Advisors
Bradstreet Clinical Research
Burleson Research Technologies (BRT)
Cambridge Biomedical
CATO Research
Celerion
Clinical Research Management Group
Clinical Supplies Management Holdings
Clinipace
A10 Clinical Solutions
ABF Pharmaceutical Services
Accell Clinical Research
Accumedix, Inc
ACM Global Laboratories
Acurian
Advanced Clinical Research (ACR)
Advanced Clinical Services LLC
Akos Urgent Care
Alcami
Alliance for Clinical Trials in Oncology
AlphaGenesis Incorporated (AGI)
Anabase
Applied Biosystems
Aptuit
ARS Clinical Trials
Aurum Clinical Research
Avitacor
AXIS Clinicals
Axon Medchem
BASi
Bio Analytical Research Corporation (BARC)
BioClinica
BioPharma Services Inc
Biostat International
Blue Sky BioServices
Bracket
Bright Pharmaceutical Services
Camargo Pharmaceutical Services
Catalent
CCS Associates
Clinical Research Consulting, Inc.
Clinical Site Services (CSS)
Clinilabs
CliniRx
Advanced Clinical Services LLC
Aptuit
Astron Research
BioClinica
Bracket
Chembiotech Laboratories
Clinlogix
Cmed
CrownBio
Ergomed
hVIVO
OPIS
Pharm-Olam
Pharmalys
Premier Research
Progressive Life Sciences
RenaSci
RTI Health Solutions
Simbec Research
Solicitors Regulation Authority
Synteract
The Clinical Trial Company
Theradex
Venn Life Sciences
ZEINCRO
Applied Healthcare Resource Management (AHRM)
ArisGloba
AXON
Biotrial
Celerion
Clinical Site Services (CSS)
ClinTec International
Congenix
Cyprotex Limited
ERT
MAPI
Orphan Reach
Pharmaceutical Development Services
PharSafer
PrimeVigilance
Quanticate
Richmond Pharmacology
Selcia
Smithers Viscient
Source BioScience Limited
TAKE Solutions
The Smerud Medical Research Group
Transcom Global
Veristat





























