The Cell and Gene Therapy Cold Chain Logistics Market Report 2023-2033: This report will prove invaluable to leading firms striving for new revenue pockets if they wish to better understand the industry and its underlying dynamics. It will be useful for companies that would like to expand into different industries or to expand their existing operations in a new region.
Favourable Initiatives and Programs for Easier Access to Cell and Gene Therapy Driving Market Growth
Patient access programs are initiatives designed to facilitate the access of patients with rare or serious diseases to cell and gene therapies. These programs offer various types of support to ensure that patients receive effective therapy in a timely manner. Support may include financial assistance, education and awareness, regulatory guidance, and transportation assistance. Due to the high cost of these therapies, affordability becomes a significant challenge for patients. To address this issue, governments and biopharmaceutical companies have implemented patient access programs that provide financial aid and reimbursement schemes, aiming to extend the reach of these therapies to patients. For examples, Novartis provide Patient Assistance NOW Program, a patient access programs for cell and gene therapy. This program offers financial assistance and support services to eligible patients receiving Kymriah and other medications from Novartis.
However, the cost associated with the storage and temperature-controlled transport of biologics is essential to maintain the integrity and effectiveness of these specialized medicines. Specifically, biologics that necessitate ultra-low temperature storage and transportation, like gene therapies and cell-based therapies, can incur significant expenses. This high cost is expected to act as a limiting factor for the growth of the cell and gene therapy cold chain logistics market.
Stringent Government Regulations to Hamper Industry Growth
Various governments have implemented stringent regulations regarding the importation of cell and gene therapeutics. Regulatory bodies require manufacturers to meet specific safety standards before granting market approval and allowing distribution. Similar rules apply to the importation of biologics, including vaccines and cell and gene therapies. Certain countries have additional regulations such as import permits, labeling requirements, storage information, and customs clearance documents for imported biologics. Logistic companies, along with their regulatory teams, must provide all necessary documentation to ensure a safe and legal process through the regulatory authorities. Moreover, such regulations can present obstacles to importing biotherapeutics into developing countries with less established regulatory frameworks, thus limiting patient access to these treatments in those regions.
What Questions Should You Ask before Buying a Market Research Report?
• How is the vaccine contract manufacturing market evolving?
• What is driving and restraining the vaccine contract manufacturing market?
• How will each vaccine contract manufacturing submarket segment grow over the forecast period and how much revenue will these submarkets account for in 2033?
• How will the market shares for each vaccine contract manufacturing submarket develop from 2023 to 2033?
• What will be the main driver for the overall market from 2023 to 2033?
• Will leading vaccine contract manufacturing markets broadly follow the macroeconomic dynamics, or will individual national markets outperform others?
• How will the market shares of the national markets change by 2033 and which geographical region will lead the market in 2033?
• Who are the leading players and what are their prospects over the forecast period?
• What are the vaccine contract manufacturing projects for these leading companies?
• How will the industry evolve during the period between 2023 and 2033? What are the implications of vaccine contract manufacturing projects taking place now and over the next 10 years?
• Is there a greater need for product commercialisation to further scale the vaccine contract manufacturing market?
• Where is the vaccine contract manufacturing market heading and how can you ensure you are at the forefront of the market?
• What are the best investment options for new product and service lines?
• What are the key prospects for moving companies into a new growth path and C-suite?
You need to discover how this will impact the vaccine contract manufacturing market today, and over the next 10 years:
• Our 322-page report provides 119 tables and 187 charts/graphs exclusively to you.
• The report highlights key lucrative areas in the industry so you can target them – NOW.
• It contains in-depth analysis of global, regional and national sales and growth.
• It highlights for you the key successful trends, changes and revenue projections made by your competitors.
This report tells you TODAY how the vaccine contract manufacturing market will develop in the next 10 years, and in line with the variations in COVID-19 economic recession and bounce. This market is more critical now than at any point over the last 10 years.
Forecasts to 2033 and other analyses reveal commercial prospects
• In addition to revenue forecasting to 2033, our new study provides you with recent results, growth rates, and market shares.
• You will find original analyses, with business outlooks and developments.
• Discover qualitative analyses (including market dynamics, drivers, opportunities, restraints and challenges), cost structure, impact of rising vaccine contract manufacturing prices and recent developments.
This report includes data analysis and invaluable insight into how COVID-19 will affect the industry and your company. Four COVID-19 recovery patterns and their impact, namely, “V”, “L”, “W” and “U” are discussed in this report.
Segments Covered in the Report
Component
• Cryogenic Shippers
• Cryogenic Storage Freezers
• Ultra-Low Freezers
• Cold Chain Management Systems
• Shipment and Storage Medium
• Cryogenic Packout Kits
• Others
Services
• Transportation
• Storage
• Packaging
Mode of Transport
• Air Transport
• Ground Transport
• Water Transports
Holding Temperature Range
• Cryogenic
• Refrigerated
• Ambient
• Others
End-users
• Biopharmaceutical & Biotechnology Companies
• Academic & Research Institutes
• Others

In addition to the revenue predictions for the overall world market and segments, you will also find revenue forecasts for five regional and xx leading national markets:
North America
• U.S.
• Canada
Europe
• Germany
• UK
• France
• Spain
• Italy
• Russia
• Rest of Europe
Asia Pacific
• China
• Japan
• India
• Australia
• South Korea
• Rest of Asia Pacific
Latin America
• Brazil
• Mexico
• Argentina
• Rest of Latin America
MEA
• GCC
• South Africa
• Rest of MEA
Need industry data? Please contact us today.
The report also includes profiles and for some of the leading companies in the Cell and Gene Therapy Cold Chain Logistics Market, 2023 to 2033, with a focus on this segment of these companies’ operations.
Leading companies and the potential for market growth
• AmerisourceBergen Corporation
• Arvato Supply Chain Solutions SE
• Be The Match BioTherapies
• BioLife Solutions, Inc.
• BioStor Sytems, Inc.
• Cardinal Health, Inc.
• Catalent Inc.
• Cryoport, Inc.
• Marken (a UPS Company)
• Polar Express Transportation
• Thermo Fisher Scientific Inc.
• Yourway Biopharma Services Company
Overall world revenue for Cell and Gene Therapy Cold Chain Logistics Market, 2023 to 2033 in terms of value the market will surpass US$1,655 million in 2023, our work calculates. We predict strong revenue growth through to 2033. Our work identifies which organizations hold the greatest potential. Discover their capabilities, progress, and commercial prospects, helping you stay ahead.
How will the Cell and Gene Therapy Cold Chain Logistics Market, 2023 to 2033 report help you?
In summary, our 320+ page report provides you with the following knowledge:
• Revenue forecasts to 2033 for Cell and Gene Therapy Cold Chain Logistics Market, 2023 to 2033 Market, with forecasts for component, services, mode of transport, holding temperature range, and end-users, each forecast at a global and regional level – discover the industry’s prospects, finding the most lucrative places for investments and revenues.
• Revenue forecasts to 2033 for five regional and 18 key national markets – See forecasts for the Cell and Gene Therapy Cold Chain Logistics Market, 2023 to 2033 market in North America, Europe, Asia-Pacific, Latin America, and MEA. Also forecasted is the market in the US, Canada, Mexico, Brazil, Germany, France, UK, Italy, China, India, Japan, and Australia among other prominent economies.
• Prospects for established firms and those seeking to enter the market – including company profiles for 12 of the major companies involved in the Cell and Gene Therapy Cold Chain Logistics Market, 2023 to 2033.
Find quantitative and qualitative analyses with independent predictions. Receive information that only our report contains, staying informed with invaluable business intelligence.
Information found nowhere else
With our new report, you are less likely to fall behind in knowledge or miss out on opportunities. See how our work could benefit your research, analyses, and decisions. Visiongain’s study is for everybody needing commercial analyses for the Cell and Gene Therapy Cold Chain Logistics Market, 2023 to 2033, market-leading companies. You will find data, trends and predictions.
To access the data contained in this document please email contactus@visiongain.com
Buy our report today Cell & Gene Therapy Cold Chain Logistics Market Report 2023-2033: Forecasts by Components (Cryogenic Shippers, Cryogenic Storage Freezers, Ultra-Low Freezers, Cold Chain Management Systems, Shipment and Storage Medium, Cryogenic Packout Kits, Others), by Services (Transportation, Storage, Packaging), by Mode of Transport (Air, Ground, Water), by Holding Temperature Range (Cryogenic, Refrigerated, Ambient, Others), by End-users (Biopharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others) AND Regional and Leading National Market Analysis PLUS Analysis of Leading Companies AND COVID-19 Impact and Recovery Pattern Analysis. Avoid missing out by staying informed – order our report now.
Visiongain is a trading partner with the US Federal Government
CCR Ref number: KD4R6
Do you have any custom requirements we can help you with? Any need for a specific country, geo region, market segment or specific company information? Contact us today, we can discuss your needs and see how we can help: contactus@visiongain.com
1 Report Overview
1.1 Objectives of the Study
1.2 Introduction to Cell and Gene Therapy Cold Chain Logistics Market
1.3 What This Report Delivers
1.4 Why You Should Read This Report
1.5 Key Questions Answered by This Analytical Report
1.6 Who is This Report for?
1.7 Methodology
1.7.1 Market Definitions
1.7.2 Market Evaluation & Forecasting Methodology
1.7.3 Data Validation
1.7.3.1 Primary Research
1.7.3.2 Secondary Research
1.8 Frequently Asked Questions (FAQs)
1.9 Associated Visiongain Reports
1.10 About Visiongain
2 Executive Summary
3 Market Overview
3.1 Key Findings
3.2 Market Dynamics
3.2.1 Market Driving Factors
3.2.1.1 High Prevalence of Cancer and Genetic Disorders
3.2.1.2 Increasing Adoption of Gene and Cell Therapies
3.2.1.3 Advancements in Storage and Transportation of Biologics
3.2.1.4 Increasing Strategic Initiatives Among Market Players
3.2.2 Market Restraining Factors
3.2.2.1 High Cost of Storage
3.2.2.2 Stringent Government Regulations
3.2.3 Market Opportunities
3.2.3.1 Strong Clinical Pipeline and Increasing Approval for Cell and Gene Therapy Products
3.2.3.2 Favourable Initiatives and Programs for Easier Access to Cell and Gene Therapy
3.3 COVID-19 Impact Analysis
3.4 Porter’s Five Forces Analysis
3.4.1 Bargaining Power of Suppliers
3.4.2 Bargaining Power of Buyers
3.4.3 Competitive Rivalry
3.4.4 Threat from Substitutes
3.4.5 Threat of New Entrants
3.5 PEST Analysis
4 Cell and Gene Therapy Cold Chain Logistics Market Analysis by Components
4.1 Key Findings
4.2 Components Segment: Market Attractiveness Index
4.3 Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Components
4.4 Cryogenic Shippers
4.4.1 Market Size by Region, 2023-2033 (US$ Million)
4.4.2 Market Share by Region, 2023 & 2033 (%)
4.5 Cryogenic Storage Freezers
4.5.1 Market Size by Region, 2023-2033 (US$ Million)
4.5.2 Market Share by Region, 2023 & 2033 (%)
4.6 Ultra-Low Freezers
4.6.1 Market Size by Region, 2023-2033 (US$ Million)
4.6.2 Market Share by Region, 2023 & 2033 (%)
4.7 Cold Chain Management Systems
4.7.1 Market Size by Region, 2023-2033 (US$ Million)
4.7.2 Market Share by Region, 2023 & 2033 (%)
4.8 Shipment and Storage Medium
4.8.1 Market Size by Region, 2023-2033 (US$ Million)
4.8.2 Market Share by Region, 2023 & 2033 (%)
4.9 Cryogenic Packout Kits
4.9.1 Market Size by Region, 2023-2033 (US$ Million)
4.9.2 Market Share by Region, 2023 & 2033 (%)
4.10 Others
4.10.1 Market Size by Region, 2023-2033 (US$ Million)
4.10.2 Market Share by Region, 2023 & 2033 (%)
5 Cell and Gene Therapy Cold Chain Logistics Market Analysis by Services
5.1 Key Findings
5.2 Services Segment: Market Attractiveness Index
5.3 Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Services
5.4 Transportation
5.4.1 Market Size by Region, 2023-2033 (US$ Million)
5.4.2 Market Share by Region, 2023 & 2033 (%)
5.5 Storage
5.5.1 Market Size by Region, 2023-2033 (US$ Million)
5.5.2 Market Share by Region, 2023 & 2033 (%)
5.6 Packaging
5.6.1 Market Size by Region, 2023-2033 (US$ Million)
5.6.2 Market Share by Region, 2023 & 2033 (%)
6 Cell and Gene Therapy Cold Chain Logistics Market Analysis by Mode of Transport
6.1 Key Findings
6.2 Mode of Transport Segment: Market Attractiveness Index
6.3 Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Mode of Transport
6.4 Air
6.4.1 Market Size by Region, 2023-2033 (US$ Million)
6.4.2 Market Share by Region, 2023 & 2033 (%)
6.5 Ground
6.5.1 Market Size by Region, 2023-2033 (US$ Million)
6.5.2 Market Share by Region, 2023 & 2033 (%)
6.6 Water
6.6.1 Market Size by Region, 2023-2033 (US$ Million)
6.6.2 Market Share by Region, 2023 & 2033 (%)
7 Cell and Gene Therapy Cold Chain Logistics Market Analysis by Holding Temperature
7.1 Key Findings
7.2 Holding Temperature Segment: Market Attractiveness Index
7.3 Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Holding Temperature
7.4 Cryogenic
7.4.1 Market Size by Region, 2023-2033 (US$ Million)
7.4.2 Market Share by Region, 2023 & 2033 (%)
7.5 Refrigerated
7.5.1 Market Size by Region, 2023-2033 (US$ Million)
7.5.2 Market Share by Region, 2023 & 2033 (%)
7.6 Ambient
7.6.1 Market Size by Region, 2023-2033 (US$ Million)
7.6.2 Market Share by Region, 2023 & 2033 (%)
7.7 Others
7.7.1 Market Size by Region, 2023-2033 (US$ Million)
7.7.2 Market Share by Region, 2023 & 2033 (%)
8 Cell and Gene Therapy Cold Chain Logistics Market Analysis by End-users
8.1 Key Findings
8.2 End-users Segment: Market Attractiveness Index
8.3 Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by End-users
8.4 Biopharmaceutical & Biotechnology Companies
8.4.1 Market Size by Region, 2023-2033 (US$ Million)
8.4.2 Market Share by Region, 2023 & 2033 (%)
8.5 Academic & Research Institutes
8.5.1 Market Size by Region, 2023-2033 (US$ Million)
8.5.2 Market Share by Region, 2023 & 2033 (%)
8.6 Others
8.6.1 Market Size by Region, 2023-2033 (US$ Million)
8.6.2 Market Share by Region, 2023 & 2033 (%)
9 Cell and Gene Therapy Cold Chain Logistics Market Analysis by Region
9.1 Key Findings
9.2 Regional Market Size Estimation and Forecast
10 North America Cell and Gene Therapy Cold Chain Logistics Market Analysis
10.1 Key Findings
10.2 North America Cell and Gene Therapy Cold Chain Logistics Market Attractiveness Index
10.3 North America Cell and Gene Therapy Cold Chain Logistics Market by Country, 2023, 2028 & 2033 (US$ Million)
10.4 North America Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Country
10.5 North America Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Components
10.6 North America Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Services
10.7 North America Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Mode of Transport
10.8 North America Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Holding Temperature
10.9 North America Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by End-users
10.10 U.S. Cell and Gene Therapy Cold Chain Logistics Market Analysis
10.11 Canada Cell and Gene Therapy Cold Chain Logistics Market Analysis
11 Europe Cell and Gene Therapy Cold Chain Logistics Market Analysis
11.1 Key Findings
11.2 Europe Cell and Gene Therapy Cold Chain Logistics Market Attractiveness Index
11.3 Europe Cell and Gene Therapy Cold Chain Logistics Market by Country, 2023, 2028 & 2033 (US$ Million)
11.4 Europe Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Country
11.5 Europe Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Components
11.6 Europe Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Services
11.7 Europe Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Mode of Transport
11.8 Europe Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Holding Temperature
11.9 Europe Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by End-users
11.10 Germany Cell and Gene Therapy Cold Chain Logistics Market Analysis
11.11 UK Cell and Gene Therapy Cold Chain Logistics Market Analysis
11.12 France Cell and Gene Therapy Cold Chain Logistics Market Analysis
11.13 Italy Cell and Gene Therapy Cold Chain Logistics Market Analysis
11.14 Spain Cell and Gene Therapy Cold Chain Logistics Market Analysis
11.15 Russia Cell and Gene Therapy Cold Chain Logistics Market Analysis
11.16 Rest of Europe Cell and Gene Therapy Cold Chain Logistics Market Analysis
12 Asia Cell and Gene Therapy Cold Chain Logistics Market Analysis
12.1 Key Findings
12.2 Asia Cell and Gene Therapy Cold Chain Logistics Market Attractiveness Index
12.3 Asia Cell and Gene Therapy Cold Chain Logistics Market by Country, 2023, 2028 & 2033 (US$ Million)
12.4 Asia Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Country
12.5 Asia Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Components
12.6 Asia Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Services
12.7 Asia Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Mode of Transport
12.8 Asia Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Holding Temperature
12.9 Asia Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by End-users
12.10 Japan Cell and Gene Therapy Cold Chain Logistics Market Analysis
12.11 China Cell and Gene Therapy Cold Chain Logistics Market Analysis
12.12 India Cell and Gene Therapy Cold Chain Logistics Market Analysis
12.13 Australia Cell and Gene Therapy Cold Chain Logistics Market Analysis
12.14 South Korea Cell and Gene Therapy Cold Chain Logistics Market Analysis
12.15 Rest of Asia Cell and Gene Therapy Cold Chain Logistics Market Analysis
13 Latin America Cell and Gene Therapy Cold Chain Logistics Market Analysis
13.1 Key Findings
13.2 Latin America Cell and Gene Therapy Cold Chain Logistics Market Attractiveness Index
13.3 Latin America Cell and Gene Therapy Cold Chain Logistics Market by Country, 2023, 2028 & 2033 (US$ Million)
13.4 Latin America Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Country
13.5 Latin America Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Components
13.6 Latin America Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Services
13.7 Latin America Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Mode of Transport
13.8 Latin America Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Holding Temperature
13.9 Latin America Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by End-users
13.10 Brazil Cell and Gene Therapy Cold Chain Logistics Market Analysis
13.11 Mexico Cell and Gene Therapy Cold Chain Logistics Market Analysis
13.12 Argentina Cell and Gene Therapy Cold Chain Logistics Market Analysis
13.13 Rest of Latin America Cell and Gene Therapy Cold Chain Logistics Market Analysis
14 MEA Cell and Gene Therapy Cold Chain Logistics Market Analysis
14.1 Key Findings
14.2 MEA Cell and Gene Therapy Cold Chain Logistics Market Attractiveness Index
14.3 MEA Cell and Gene Therapy Cold Chain Logistics Market by Country, 2023, 2028 & 2033 (US$ Million)
14.4 MEA Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Country
14.5 MEA Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Components
14.6 MEA Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Services
14.7 MEA Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Mode of Transport
14.8 MEA Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by Holding Temperature
14.9 MEA Cell and Gene Therapy Cold Chain Logistics Market Size Estimation and Forecast by End-users
14.10 GCC Cell and Gene Therapy Cold Chain Logistics Market Analysis
14.11 South Africa Cell and Gene Therapy Cold Chain Logistics Market Analysis
14.12 Rest of MEA Cell and Gene Therapy Cold Chain Logistics Market Analysis
15 Company Profiles
15.1 Company Share Analysis, 2022
15.2 Strategic Outlook
15.3 AmerisourceBergen Corporation
15.3.1 Company Snapshot
15.3.2 Company Overview
15.3.3 Financial Analysis
15.3.3.1 Net Revenue, 2017-2022
15.3.3.2 Regional Market Shares, 2022
15.3.3.3 Segment Market Shares, 2022
15.3.4 Product Benchmarking
15.3.5 Strategic Outlook
15.4 Arvato Supply Chain Solutions SE
15.4.1 Company Snapshot
15.4.2 Company Overview
15.4.3 Product Benchmarking
15.4.4 Strategic Outlook
15.5 Be The Match BioTherapies
15.5.1 Company Snapshot
15.5.2 Company Overview
15.5.3 Product Benchmarking
15.5.4 Strategic Outlook
15.6 BioLife Solutions, Inc
15.6.1 Company Snapshot
15.6.2 Company Overview
15.6.3 Financial Analysis
15.6.3.1 Net Revenue, 2017-2022
15.6.3.2 Segment Market Shares, 2022
15.6.4 Product Benchmarking
15.6.5 Strategic Outlook
15.7 Cardinal Health Inc
15.7.1 Company Snapshot
15.7.2 Company Overview
15.7.3 Financial Analysis
15.7.3.1 Net Revenue, 2017-2022
15.7.3.2 Regional Market Shares, 2022
15.7.3.3 Segment Market Shares, 2022
15.7.4 Product Benchmarking
15.7.5 Strategic Outlook
15.8 Catalent, Inc.
15.8.1 Company Snapshot
15.8.2 Company Overview
15.8.3 Financial Analysis
15.8.3.1 Net Revenue, 2017-2022
15.8.3.2 R&D, 2017-2022
15.8.3.3 Regional Market Shares, 2022
15.8.3.4 Segment Market Shares, 2022
15.8.4 Product Benchmarking
15.8.5 Strategic Outlook
15.9 Cryoport, Inc.
15.9.1 Company Snapshot
15.9.2 Company Overview
15.9.3 Financial Analysis
15.9.3.1 Net Revenue, 2017-2022
15.9.3.2 Regional Market Shares, 2022
15.9.3.3 Segment Market Shares, 2022
15.9.4 Product Benchmarking
15.9.5 Strategic Outlook
15.10 BioStor Sytems, Inc.
15.10.1 Company Snapshot
15.10.2 Company Overview
15.10.3 Product Benchmarking
15.11 Marken (a UPS Company)
15.11.1 Company Snapshot
15.11.2 Company Overview
15.11.3 Financial Analysis
15.11.3.1 Net Revenue, 2017-2022
15.11.3.2 Regional Market Shares, 2022
15.11.3.3 Segment Market Shares, 2022
15.11.4 Product Benchmarking
15.11.5 Strategic Outlook
15.12 Polar Express Transportation
15.12.1 Company Snapshot
15.12.2 Company Overview
15.12.3 Product Benchmarking
15.13 Thermo Fisher Scientific Inc.
15.13.1 Company Snapshot
15.13.2 Company Overview
15.13.3 Financial Analysis
15.13.3.1 Net Revenue, 2017-2022
15.13.3.2 R&D, 2017-2022
15.13.3.3 Regional Market Shares, 2022
15.13.3.4 Segment Market Shares, 2022
15.13.4 Product Benchmarking
15.13.5 Strategic Outlook
15.14 Yourway Biopharma Services Company
15.14.1 Company Snapshot
15.14.2 Company Overview
15.14.3 Product Benchmarking
16 Conclusion and Recommendations
16.1 Concluding Remarks from Visiongain
16.2 Recommendations for Market Players
List of Tables
Table 1 Cell and Gene Therapy Cold Chain Logistics Market Snapshot, 2023 & 2033 (US$ Million, CAGR %)
Table 2 Approved Cell and Gene therapies
Table 3 Clinical Pipeline: Cell and Gene Therapy Products
Table 4 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%): “V” Shaped Recovery
Table 5 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%): “U” Shaped Recovery
Table 6 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%): “W” Shaped Recovery
Table 7 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%): “L” Shaped Recovery
Table 8 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Components, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 9 Cryogenic Shippers Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 10 Cryogenic Storage Freezers Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 11 Ultra-Low Freezers Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 12 Cold Chain Management Systems Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 13 Shipment and Storage Medium Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 14 Cryogenic Packout Kits Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 15 Others Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 16 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Services, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 17 Transportation Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 18 Storage Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 19 Packaging Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 20 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Mode of Transport, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 21 Air Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 22 Ground Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 23 Water Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 24 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Holding Temperature, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 25 Cryogenic Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 26 Refrigerated Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 27 Ambient Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 28 Others Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 29 Cell and Gene Therapy Cold Chain Logistics Market Forecast by End-users, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 30 Biopharmaceutical & Biotechnology Companies Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 31 Academic & Research Institutes Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 32 Others Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 33 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Region, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 34 North America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Country, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 35 North America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Components, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 36 North America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Services, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 37 North America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Mode of Transport, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 38 North America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Holding Temperature, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 39 Cell and Gene Therapy Cold Chain Logistics Market Forecast by End-users, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 40 U.S. Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 41 Canada Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 42 Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast by Country, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 43 Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast by Components, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 44 Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast by Services, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 45 Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast by Mode of Transport, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 46 Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast by Holding Temperature, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 47 Cell and Gene Therapy Cold Chain Logistics Market Forecast by End-users, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 48 Germany Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 49 UK Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 50 France Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 51 Italy Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 52 Spain Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 53 Russia Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 54 Rest of Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 55 Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast by Country, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 56 Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast by Components, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 57 Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast by Services, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 58 Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast by Mode of Transport, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 59 Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast by Holding Temperature, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 60 Cell and Gene Therapy Cold Chain Logistics Market Forecast by End-users, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 61 Japan Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 62 China Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 63 India Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 64 Australia Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 65 South Korea Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 66 Rest of Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 67 Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Country, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 68 Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Components, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 69 Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Services, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 70 Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Mode of Transport, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 71 Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Holding Temperature, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 72 Cell and Gene Therapy Cold Chain Logistics Market Forecast by End-users, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 73 Brazil Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 74 Mexico Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 75 Argentina Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 76 Rest of Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 77 MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast by Country, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 78 MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast by Components, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 79 MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast by Services, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 80 MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast by Mode of Transport, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 81 MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast by Holding Temperature, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 82 Cell and Gene Therapy Cold Chain Logistics Market Forecast by End-users, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 83 GCC Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 84 South Africa Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 85 Rest of MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR%, CAGR%)
Table 86 Strategic Outlook
Table 87 AmerisourceBergen Corporation: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 88 AmerisourceBergen Corporation: Product Benchmarking
Table 89 AmerisourceBergen Corporation Strategic Outlook
Table 90 Arvato Supply Chain Solutions SE: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 91 Arvato Systems: Product Benchmarking
Table 92 Arvato Systems Strategic Outlook
Table 93 Be The Match BioTherapies: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 94 Be The Match BioTherapies: Product Benchmarking
Table 95 Be The Match BioTherapies Strategic Outlook
Table 96 BioLife Solution Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 97 BioLife Solution Inc.: Product Benchmarking
Table 98 BioLife Solution Inc. Strategic Outlook
Table 99 Cardinal Health, Inc: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 100 Cardinal Health, Inc: Product Benchmarking
Table 101 Cardinal Health, Inc Strategic Outlook
Table 102 Catalent Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 103 Catalent Inc.: Product Benchmarking
Table 104 Catalent Inc. Strategic Outlook
Table 105 Cryoport: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 106 Cryoport: Product Benchmarking
Table 107 Cryoport Strategic Outlook
Table 108 BioStor Sytems, Inc.: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 109 BioStor Sytems, Inc.: Product Benchmarking
Table 110 Marken (a UPS Company): Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 111 Marken (a UPS Company): Product Benchmarking
Table 112 Marken (a UPS Company) Strategic Outlook
Table 113 Polar Express Transportation: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 114 Polar Express Transportation: Product Benchmarking
Table 115 Thermo Fisher Scientific: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 116 Thermo Fisher Scientific: Product Benchmarking
Table 117 Thermo Fisher Scientific Strategic Outlook
Table 118 Yourway Biopharma Services Company: Key Details, (CEO, HQ, Revenue, Founded, No. of Employees, Company Type, Website, Business Segment)
Table 119 Yourway Biopharma Services Company: Product Benchmarking
List of Figures
Figure 1 Cell and Gene Therapy Cold Chain Logistics Market Segmentation
Figure 2 Cell and Gene Therapy Cold Chain Logistics Market by Component: Market Attractiveness Index
Figure 3 Cell and Gene Therapy Cold Chain Logistics Market by Services: Market Attractiveness Index
Figure 4 Cell and Gene Therapy Cold Chain Logistics Market by Mode of Transport Market Attractiveness Index
Figure 5 Cell and Gene Therapy Cold Chain Logistics Market by Holding Temperature Range: Market Attractiveness Index
Figure 6 Cell and Gene Therapy Cold Chain Logistics Market by End-users: Market Attractiveness Index
Figure 7 Cell and Gene Therapy Cold Chain Logistics Market Attractiveness Index by Region
Figure 8 Cell and Gene Therapy Cold Chain Logistics Market: Market Dynamics
Figure 9 Cell and Gene Therapy Cold Chain Logistics Market by Region, 2023-2033 (US$ Million, AGR %): “V” Shaped Recovery
Figure 10 Cell and Gene Therapy Cold Chain Logistics Market by Region, 2023-2033 (US$ Million, AGR %): “U” Shaped Recovery
Figure 11 Cell and Gene Therapy Cold Chain Logistics Market by Region, 2023-2033 (US$ Million, AGR %): “W” Shaped Recovery
Figure 12 Cell and Gene Therapy Cold Chain Logistics Market by Region, 2023-2033 (US$ Million, AGR %): “L” Shaped Recovery
Figure 13 Cell and Gene Therapy Cold Chain Logistics Market: Porter’s Five Forces Analysis
Figure 14 Cell and Gene Therapy Cold Chain Logistics Market: PEST Analysis
Figure 15 Cell and Gene Therapy Cold Chain Logistics Market by Components: Market Attractiveness Index
Figure 16 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Components, 2023-2033 (US$ Million, AGR %)
Figure 17 Cell And Gene Therapy Cold Chain Logistics Share Forecast by Components, 2023, 2028, 2033 (%)
Figure 18 Cryogenic Shippers Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 19 Cryogenic Shippers Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 20 Cryogenic Storage Freezers Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 21 Cryogenic Storage Freezers Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 22 Ultra-Low Freezers Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 23 Ultra-Low Freezers Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 24 Cold Chain Management Systems Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 25 Cold Chain Management Systems Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 26 Shipment and Storage Medium Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 27 Shipment and Storage Medium Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 28 Cryogenic Packout Kits Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 29 Cryogenic Packout Kits Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 30 Others Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 31 Others Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 32 Cell and Gene Therapy Cold Chain Logistics Market by Services: Market Attractiveness Index
Figure 33 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Services, 2023-2033 (US$ Million, AGR %)
Figure 34 Cell And Gene Therapy Cold Chain Logistics Share Forecast by Services, 2023, 2028, 2033 (%)
Figure 35 Transportation Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 36 Transportation Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 37 Storage Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 38 Storage Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 39 Packaging Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 40 Packaging Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 41 Cell and Gene Therapy Cold Chain Logistics Market by Mode of Transport: Market Attractiveness Index
Figure 42 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Mode of Transport, 2023-2033 (US$ Million, AGR %)
Figure 43 Cell And Gene Therapy Cold Chain Logistics Share Forecast by Mode of Transport, 2023, 2028, 2033 (%)
Figure 44 Air Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 45 Air Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 46 Ground Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 47 Ground Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 48 Water Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 49 Water Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 50 Cell and Gene Therapy Cold Chain Logistics Market by Holding Temperature: Market Attractiveness Index
Figure 51 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Holding Temperature, 2023-2033 (US$ Million, AGR %)
Figure 52 Cell And Gene Therapy Cold Chain Logistics Share Forecast by Holding Temperature, 2023, 2028, 2033 (%)
Figure 53 Cryogenic Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 54 Cryogenic Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 55 Refrigerated Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 56 Refrigerated Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 57 Ambient Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 58 Ambient Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 59 Others Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 60 Others Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 61 Cell and Gene Therapy Cold Chain Logistics Market by End-users: Market Attractiveness Index
Figure 62 Cell and Gene Therapy Cold Chain Logistics Market Forecast by End-users, 2023-2033 (US$ Million, AGR %)
Figure 63 Cell And Gene Therapy Cold Chain Logistics Share Forecast by End-users, 2023, 2028, 2033 (%)
Figure 64 Biopharmaceutical & Biotechnology Companies Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 65 Biopharmaceutical & Biotechnology Companies Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 66 Academic & Research Institutes Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 67 Academic & Research Institutes Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 68 Others Segment Market Forecast by Region, 2023-2033 (US$ Million, AGR %)
Figure 69 Others Segment Market Share Forecast by Region, 2023 & 2033 (%)
Figure 70 Cell and Gene Therapy Cold Chain Logistics Market Forecast by Region 2023 and 2033 (Revenue, CAGR%)
Figure 71 Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Region 2023, 2028, 2033 (%)
Figure 72 Cell and Gene Therapy Cold Chain Logistics Market by Region, 2023-2033 (US$ Million, AGR %)
Figure 73 North America Cell and Gene Therapy Cold Chain Logistics Market Attractiveness Index
Figure 74 North America Cell and Gene Therapy Cold Chain Logistics Market by Region, 2023, 2028 & 2033 (US$ Million)
Figure 75 North America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Country, 2023-2033 (US$ Million, AGR %)
Figure 76 North America Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Country, 2023 & 2033 (%)
Figure 77 North America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Components, 2023-2033 (US$ Million, AGR %)
Figure 78 North America Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Components, 2023 & 2033 (%)
Figure 79 North America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Services, 2023-2033 (US$ Million, AGR %)
Figure 80 North America Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Services, 2023 & 2033 (%)
Figure 81 North America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Mode of Transport, 2023-2033 (US$ Million, AGR %)
Figure 82 North America Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Mode of Transport, 2023 & 2033 (%)
Figure 83 North America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Holding Temperature, 2023-2033 (US$ Million, AGR %)
Figure 84 North America Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Holding Temperature, 2023 & 2033 (%)
Figure 85 North America Cell and Gene Therapy Cold Chain Logistics Market Forecast by End-users, 2023-2033 (US$ Million, AGR %)
Figure 86 North America Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by End-users, 2023 & 2033 (%)
Figure 87 U.S. Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 88 Canada Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 89 Europe Cell and Gene Therapy Cold Chain Logistics Market Attractiveness Index
Figure 90 Europe Cell and Gene Therapy Cold Chain Logistics Market by Region, 2023, 2028 & 2033 (US$ Million)
Figure 91 Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast by Country, 2023-2033 (US$ Million, AGR %)
Figure 92 Europe Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Country, 2023 & 2033 (%)
Figure 93 Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast by Components, 2023-2033 (US$ Million, AGR %)
Figure 94 Europe Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Components, 2023 & 2033 (%)
Figure 95 Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast by Services, 2023-2033 (US$ Million, AGR %)
Figure 96 Europe Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Services, 2023 & 2033 (%)
Figure 97 Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast by Mode of Transport, 2023-2033 (US$ Million, AGR %)
Figure 98 Europe Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Mode of Transport, 2023 & 2033 (%)
Figure 99 Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast by Holding Temperature, 2023-2033 (US$ Million, AGR %)
Figure 100 Europe Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Holding Temperature, 2023 & 2033 (%)
Figure 101 Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast by End-users, 2023-2033 (US$ Million, AGR %)
Figure 102 Europe Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by End-users, 2023 & 2033 (%)
Figure 103 Germany Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 104 UK Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 105 France Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 106 Italy Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 107 Spain Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 108 Russia Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 109 Rest of Europe Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 110 Asia Cell and Gene Therapy Cold Chain Logistics Market Attractiveness Index
Figure 111 Asia Cell and Gene Therapy Cold Chain Logistics Market by Region, 2023, 2028 & 2033 (US$ Million)
Figure 112 Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast by Country, 2023-2033 (US$ Million, AGR %)
Figure 113 Asia Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Country, 2023 & 2033 (%)
Figure 114 Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast by Components, 2023-2033 (US$ Million, AGR %)
Figure 115 Asia Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Components, 2023 & 2033 (%)
Figure 116 Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast by Services, 2023-2033 (US$ Million, AGR %)
Figure 117 Asia Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Services, 2023 & 2033 (%)
Figure 118 Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast by Mode of Transport, 2023-2033 (US$ Million, AGR %)
Figure 119 Asia Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Mode of Transport, 2023 & 2033 (%)
Figure 120 Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast by Holding Temperature, 2023-2033 (US$ Million, AGR %)
Figure 121 Asia Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Holding Temperature, 2023 & 2033 (%)
Figure 122 Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast by End-users, 2023-2033 (US$ Million, AGR %)
Figure 123 Asia Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by End-users, 2023 & 2033 (%)
Figure 124 Japan Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 125 China Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 126 India Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 127 Australia Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 128 South Korea Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 129 Rest of Asia Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 130 Latin America Cell and Gene Therapy Cold Chain Logistics Market Attractiveness Index
Figure 131 Latin America Cell and Gene Therapy Cold Chain Logistics Market by Region, 2023, 2028 & 2033 (US$ Million)
Figure 132 Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Country, 2023-2033 (US$ Million, AGR %)
Figure 133 Latin America Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Country, 2023 & 2033 (%)
Figure 134 Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Components, 2023-2033 (US$ Million, AGR %)
Figure 135 Latin America Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Components, 2023 & 2033 (%)
Figure 136 Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Services, 2023-2033 (US$ Million, AGR %)
Figure 137 Latin America Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Services, 2023 & 2033 (%)
Figure 138 Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Mode of Transport, 2023-2033 (US$ Million, AGR %)
Figure 139 Latin America Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Mode of Transport, 2023 & 2033 (%)
Figure 140 Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast by Holding Temperature, 2023-2033 (US$ Million, AGR %)
Figure 141 Latin America Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Holding Temperature, 2023 & 2033 (%)
Figure 142 Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast by End-users, 2023-2033 (US$ Million, AGR %)
Figure 143 Latin America Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by End-users, 2023 & 2033 (%)
Figure 144 Brazil Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 145 Mexico Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 146 Argentina Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 147 Rest of Latin America Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 148 MEA Cell and Gene Therapy Cold Chain Logistics Market Attractiveness Index
Figure 149 MEA Cell and Gene Therapy Cold Chain Logistics Market by Region, 2023, 2028 & 2033 (US$ Million)
Figure 150 MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast by Country, 2023-2033 (US$ Million, AGR %)
Figure 151 MEA Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Country, 2023 & 2033 (%)
Figure 152 MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast by Components, 2023-2033 (US$ Million, AGR %)
Figure 153 MEA Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Components, 2023 & 2033 (%)
Figure 154 MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast by Services, 2023-2033 (US$ Million, AGR %)
Figure 155 MEA Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Services, 2023 & 2033 (%)
Figure 156 MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast by Mode of Transport, 2023-2033 (US$ Million, AGR %)
Figure 157 MEA Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Mode of Transport, 2023 & 2033 (%)
Figure 158 MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast by Holding Temperature, 2023-2033 (US$ Million, AGR %)
Figure 159 MEA Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by Holding Temperature, 2023 & 2033 (%)
Figure 160 MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast by End-users, 2023-2033 (US$ Million, AGR %)
Figure 161 MEA Cell and Gene Therapy Cold Chain Logistics Market Share Forecast by End-users, 2023 & 2033 (%)
Figure 162 GCC Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 163 South Africa Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
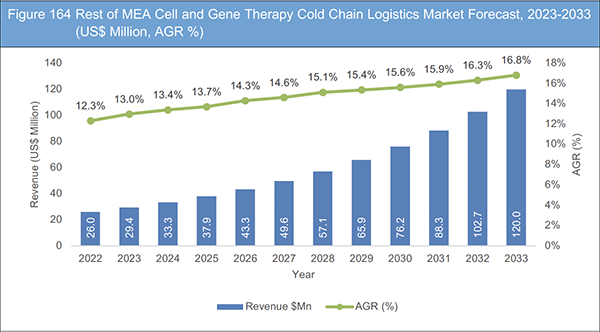
Figure 164 Rest of MEA Cell and Gene Therapy Cold Chain Logistics Market Forecast, 2023-2033 (US$ Million, AGR %)
Figure 165 Cell and Gene Therapy Cold Chain Logistics Market: Company Share Analysis, 2022
Figure 166 AmerisourceBergen Corporation: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 167 AmerisourceBergen Corporation: Regional Market Shares, 2022
Figure 168 AmerisourceBergen Corporation: Business Segment Market Shares, 2022
Figure 169 BioLife Solution Inc.: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 170 BioLife Solution Inc: Business Segment Market Shares, 2022
Figure 171 Cardinal Health, Inc: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 172 Cardinal Health, Inc: Regional Market Shares, 2022
Figure 173 Cardinal Health, Inc: Business Segment Market Shares, 2022
Figure 174 Catalent Inc.: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 175 Catalent Inc.: R&D, 2017-2022 (US$ Million, AGR%)
Figure 176 Catalent Inc.: Regional Market Shares, 2022
Figure 177 Catalent Inc.: Business Segment Market Shares, 2022
Figure 178 Cryoport: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 179 Cryoport: Regional Market Shares, 2022
Figure 180 Cryoport: Business Segment Market Shares, 2022
Figure 181 Marken (a UPS Company): Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 182 Marken (a UPS Company): Regional Market Shares, 2022
Figure 183 Marken (a UPS Company): Business Segment Market Shares, 2022
Figure 184 Thermo Fisher Scientific: Net Revenue, 2017-2022 (US$ Million, AGR%)
Figure 185 Thermo Fisher Scientific: R&D, 2017-2022 (US$ Million, AGR%)
Figure 186 Thermo Fisher Scientific: Regional Market Shares, 2022
Figure 187 Thermo Fisher Scientific: Business Segment Market Shares, 2022
List of Companies Profiled in the Report
AmerisourceBergen Corporation
Arvato Supply Chain Solutions SE
Be The Match BioTherapies
BioLife Solutions, Inc.
BioStor Sytems, Inc.
Cardinal Health, Inc.
Catalent Inc.
Cryoport, Inc.
Marken (a UPS Company)
Polar Express Transportation
Thermo Fisher Scientific Inc.
Yourway Biopharma Services Company
List of Other Companies Mentioned in the Report
ABL Research and Development (R&D) Biotech
Arsenal Biosciences
Astellas Pharma
Atara Biotherapeutics, Inc.
B Medical Systems
Bayer AG
Bendcare group purchasing organization (CPO-GPO)
BioLife Plasma Services
BioNTech SE
Bomi Group
Carina Biotech
CARsgen Therapeutics
Cedra Express
Cell and Gene Therapy Catapult
Cell Matters
Cell&Co BioServices
CEVEC Pharmaceuticals
Chimeric Therapeutics
Chronicled
CIBMTR Clinical Research Organization Services
COAVE Therapeutics
Curocell Inc.
Cytiva
DB Schenker
DHL
Ember Technologies, Inc.
Envirotainer
Evotec SE
ExCellThera
FedEx
Freeline Therapeutics
Genespire
Genethon
Gilead Sciences
Grifols
Hanjin
Homology Medicines
IASO Biotherapeutics/Invent
Institut de la Vision
Institut Imagine
ION Solutions
JW Therapeutics
Kite (Gilead Sciences, Inc.)
Körber
Logista
Merck KGaA
Mitsubishi Logistics Corporation (MLC)
Moderna, Inc.
MVE Biological Solutions
Novartis
Novavax
Palantir Technologies Inc.
Pfizer Inc.
PharmaLex Holding GmbH
Porton Advanced Solutions
PPD, Inc.
ReiThera
Rigenerand Srla
Sarepta Therapeutics, Inc.
SaudiVax
SCG Cell Therapy
Sensitech EMEA B.V.
Sirion Biotech
Sofinnova Partners
Spark Therapeutics
Symbiosis Pharmaceutical Services
Syneos Health
Takeda Pharmaceutical Company Limited
The Binding Site Group
TrakCel Ltd.
Transportes El Mosca
Ultragenyx Pharmaceutical
Vineti, Inc.
Viralgen
Walgreens Boots Alliance
WuXi AppTec
Yinjia (Shanghai) Biosciences
List of Associations Mentioned in the Report
Argentina: National Administration on Drugs, Foods, and Medical Devices (ANMAT)
Cape Biomanufacturing Industry Development Centre (CBIDC)
Center for Regenerative Medicine, Venezuela
Chulalongkorn University
Dubai Stem Cell Congress
Egypt's Ministry of Health and Population
Hortman Stem Cell Laboratory
International Society for Cell and Gene Therapy
Korean Ministry of Food and Drug Safety (MFDS)
Meary Center
Medical Research Future Fund
National Medical Products Administration (NMPA)
National Research Council
Singapore Economic Development Board (SEDB)
UK Research and Innovation (UKRI)
World Health Organization (WHO)





























